Endo & Exothermic Rxns - WaylandHighSchoolChemistry
advertisement

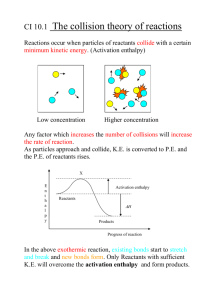
Endo & Exothermic Rxns • I know the factors that determine whether a reaction will occur. • I know the difference between endo and exothermic reactions. • I can identify the components of a potential energy diagram. Reactions • In order for a reaction to occur the molecules must: – Collide – with the proper orientation – and set energy. Lighting a match In order to light a match: • The match must collide with the side of the box that is rough. • The head of the match must be oriented to face the box • The strike must have enough energy. Activation Energy • The set energy required to get reaction going. • The larger the amount of activation energy the slower the reaction. DE1 > DE2 therefore the second reaction is faster than the first. Endothermic • Process that absorbs heat from the surroundings – Feels cold because your hand (body) is part of the surroundings. • Heat, q is positive – q stands for the quantity of heat. • Chemical potential energy of reactants is lower than the products. ~4kJ Potential energy diagram ~1.5kJ ~0.5kJ ~3.5kJ The y-axis says energy now, could also say potential energy or enthalpy. They all mean the same thing. How much energy is stored in the bonds of the molecules! The transition state is where the reactants are • (Ea)f is the activation energy for transitioning into the products. This is an activated complex because it has bonds the forward reaction. breaking and forming so it could do either way. • (E ) is the activation energy for a r the reverse reaction. • DH is the change in enthalpy. The difference between the products and the reactants. DH is also called the heat of reaction. It is the amount of heat/energy absorbed or released during a reaction. • Notice that the (Ea)f is equal to the sum of the (Ea)r and DH. • Say (Ea)f is equal to 100 kJ and DH is equal to 80 kJ. What then would be the (Ea)r? Catalyst • A species that speeds up the rate of reaction without itself being consumed. • Lowers the activation energy required to get the reaction going. Notice that the level of where the reactants start and where the products end do not change! The only thing that changes is the amount of activation energy required to get the reaction going. Exothermic Reactions • Process in which energy is released to the surroundings. – Feels hot because your hand (body) is part of the surroundings. • Heat, q is negative – Scientists needed a way to tell whether the energy was absorbed or released. It was decided to make the energy released negative • Chemical potential energy of reactants is higher than the products. Potential energy diagram About 4 kJ About 1.5 kJ Heat of rxn, DH ~3.5 kJ ~0.5 kJ Notice that now (Ea)f + DH = (Ea)r. So if (Ea)f = 50kJ and DH = 50kJ, what does (Ea)r = ? Your turn… Label each arrow Your turn… Activation energy of forward reaction because it starts at the reactant level and goes to the peak of the transition state. Change in enthalpy or Heat of reaction because it goes from the reactants to products. Enthalpy of activated complex because it goes from 0 to the peak of the transition state. Enthalpy of reactants because it goes from 0 to the reactants. Your turn take 2 2 Activation energy of forward reaction because goes from reactants to peak of transition state Activation energy of reverse reaction because goes from products to peak of transition state 5 DH, or heat of reaction because it goes from the reactant level to product level Enthalpy of reactants because goes from 0 to reactants 3 Enthalpy of activated complex or transition state because goes from 0 to peak Enthalpy of products because goes from 0 to products Differences in this PE diagram? There are actually 3 rxns occurring one after the other. The net result the combination of all 3 rxns. Notice that the activation energy of the first reaction is that greatest, that is why that reaction is labeled slow. worksheet • Complete the worksheet • Check your answers • Complete the online worksheet.