Thermodynamics Conceptual Physics Test
Name __________________________________________ Period______Date_____________
Conceptual Physics- Ch. 21, 22, 23 Test (25 pts.)
Multiple Choice
Identify the choice that best completes the statement or answers the question.
__c__ 1. Evaporation takes place when matter changes from a a. solid to a liquid. b. solid to a gas. c. liquid to a gas. d. gas to a liquid.
__b__ 2. Pigs roll in the mud a. to thermally insulate themselves. b. to keep their skin wet so evaporation can occur. c. because the darker color of mud makes them warmer. d. none of the above
__a__ 3. Condensation occurs when matter changes from a a. gas to a liquid. b. solid to a gas. c. solid to a liquid. d. liquid to a gas.
__a__ 4. When water freezes, it a. absorbs energy. b. gives off energy. c. neither gives off nor absorbs energy.
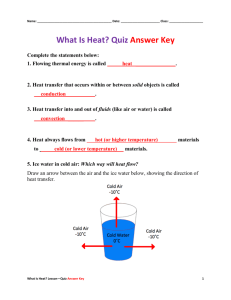
__d__ 5. On a humid day, water forms on the outside of a glass of ice water. This phenomenon occurs mainly because of a. water goes through tiny holes in the class b. water spills over the side of the class c. evaporation. d. condensation
__b__ 6. Heat travels from the sun to Earth by a. conduction. b. radiation. c. convection.
__d__ 7. Energy transfer by convection is primarily restricted to a. gases. b. liquids. c. solids. d. insulation. d. fluids.
__a__ 8. A piece of metal will feel colder than a piece of wood at the same temperature. Why? a. Metals, in general, are good heat conductors. b. Metals are colder than wood. c. Wood, in general, is a poor insulator. d. Metal atoms are moving more slowly, on the average, than wood atoms.
__a__ 9. Which temperature scale labels the freezing point of water at 0 degrees? a. Celsius b. Caloric c. Kelvin d. Fahrenheit
__c__ 10. Heat is the _____. a. average amount of energy per molecule contained in an object b. total amount of energy contained in an object c. energy transferred between objects because of a temperature difference d. amount of energy all the molecules have e. all of the above
__b__ 11.
The graph at right shows the temperature of a sample of water
as it is heated at a constant rate.
Which of the following conclusions is best supported by the information in the graph? a.
The water boils between points X and Y and condenses between points Y and Z. b.
The average kinetic energy of the water molecules increases between points X and Y and stays the same between points Y and Z. c.
The water evaporates between points X and Y and the average kinetic energy of the water molecules increases between points Y and Z. d.
The average kinetic energy of the water molecules remains the same between points X and Y and the water boils between points Y and
Z.
The table below shows data from a heating experiment.
Metal Heat Added (J) Mass of Metal (g) Change in Temperature (°C) copper iron lead
3000
3000
3000
100
100
100
77
64
231 silver 3000 100 130
__a__ 12. Which of the following conclusions is supported by the data in the table? a.
A given mass of silver requires less heat to change its temperature
1°C than an equal mass of iron.
b.
A given mass of silver requires less heat to change its temperature
1°C than an equal mass of lead.
c.
A given mass of copper requires less heat to change its temperature
1°C than an equal mass of lead.
d.
A given mass of copper requires less heat to change its temperature
1°C than an equal mass of silver .
__c__ 13. The reason an ocean's temperature doesn't vary much from one season to the next is that _____. a. oceans are located in mild regions of the Earth b. oceans are located next to large land areas c. water has a high specific heat capacity d. water has a low specific heat capacity e. there are a lot of fish in the oceans
__a__ 14. Sunlight heats a dark rock. A snake then moves onto the rock in order to increase its body temperature.Which of the following sequences represents how heat energy is transferred from the Sun to the rock to the snake? a. radiation then conduction b. conduction then radiation c. radiation then convection d. convection then radiation
__a__ 15. The lowest possible temperature in nature is a. –273 degrees C. b. 4 K. c. 0 degrees C.
Short Answer
16. (2 pts.) Explain a convection current and how it works. convection works by movement of air . . .
17. (2 pts.) Explain why bridges have metal gaps in them.
Gap found on a bridge to allow for thermal expansion
18. (6 pts.) On a hot day, a student places a glass of cold lemonade on a table outdoors. After a few minutes, water droplets have formed on the outside of the glass. a.
Is energy absorbed or released by the cold lemonade? Explain your answer.
Energy is absorbed by the lemonade. Condensation will send heat into the glass of lemonade.
b.
Compare average kinetic energy for the air molecules and lemonade molecules when the student first places the lemonade outdoors. Explain your answer. .
The average kinetic energy (temperature) of the air molecules is higher than the average kinetic energyo f the lemonade, because it is warmer.
c.
Explain how and why the water droplets form on the outside of the glass.
As the air cools, the water vapor it contains condenses.