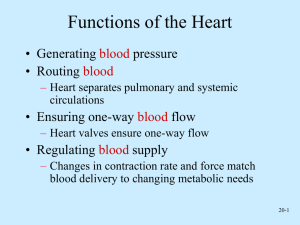
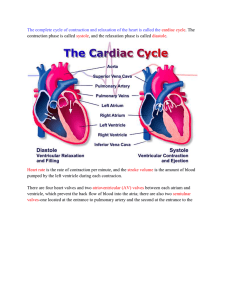
THE CARDIOVASCULAR SYSTEM: THE HEART
advertisement

THE CARDIOVASCULAR SYSTEM: THE HEART HEART LOCATION • Size, Location, and Orientation: – The heart is the size of a fist and weighs 250-300 grams – The heart is found in mediastinum and two-thirds lies left of the midsternal line – The base is directed toward the right shoulder and the apex points toward the left hip HEART LOCATION Coverings of the Heart • The heart is enclosed in a double-walled sac called the pericardium: – The loosely fitting superficial part of this sac is the fibrous pericardium (tough, dense connective tissue) • • • • Deep to fibrous pericardium is the serous pericardium: – Thin, slippery, two-layer serous membrane • • • • Protects the heart Anchors it to surrounding structures Prevents overfilling of the heart with blood The parietal pericardium lines the internal surface of the fibrous pericardium Then parietal pericardium turns inferiorly and continues over the external heart surface as the visceral pericardium, or epicardium, which is an integral part of the heart wall Between the parietal and visceral layers is the slitlike pericardial cavity, which contains a film of serous fluid The serous membranes, lubricated by the fluid, glide smoothly past one another during heart activity, allowing the mobile heart to work in a relatively friction-free environment Coverings of the Heart HOMEOSTATIC IMBALANCE • Pericarditis: inflammation of the pericardium – Hinders production of serous fluid and roughens the serous membrane surface – Heart rubs against its pericardial sac creating a creaking noise (pericardial friction rub) that can be heard with a stethoscope – Deep pain to the sternum – Pericardia stick together and impede heart activity – Severe cases: • Large amounts of inflammatory fluid seeps into the pericardial cavity compressing the heart, limiting its ability to pump blood (cardiac tamponade) – Treated by inserting a syringe into the pericardial cavity and draining off the excess fluid Layers of the Heart • • • • The heart wall is composed of three layers, all richly supplied with blood vessels Superficial epicardium is the visceral layer of the serous pericardium – Often infiltrated with fat, especially in older people Middle layer, myocardium,is composed mainly of cardiac muscle and forms the bulk of the heart – It is the layer that contracts – Cardiac muscle cells are tethered to one another by crisscrossing connective tissue fibers and arranged in spiral or circular bundles reinforcing the myocardium internally and anchors the cardiac muscle fibers The third layer, the endocardium (squamous epithelium), lines the chambers of the heart and is continuous with the endothelial linings of the blood vessels leaving and entering the heart HEART LAYERS CARDIAC MUSCLE CHAMBERS and ASSOCIATED GREAT VESSELS • Heart has four chambers: – Two superior atria – Two inferior ventricles • Internal partition that divides the heart longitudinally is called the interatrial septum where it separates the atria, and the interventricular septum where it separates the ventricles • Right ventricle forms most of the anterior surface of the heart • Left ventricle dominates the infero-posterior aspect of the heart and forms the heart apex CHAMBERS and ASSOCIATED GREAT VESSELS • Two grooves visible on the heart surface indicate the boundaries of its four chambers and carry the blood vessels supplying the myocardium • The atrioventricular groove, or coronary sulcus, encircles the junction of the atria and ventricles like a crown CHAMBERS and ASSOCIATED GREAT VESSELS • The anterior interventricular sulcus, cradling the anterior interventricular artery, marks the anterior position of the septum separating the right and left ventricles • It continues as the posterior interventricular sulcus, which provides a similar landmark on the heart’s posteroinferior surface CHAMBERS and ASSOCIATED GREAT VESSELS CHAMBERS and ASSOCIATED GREAT VESSELS HEART ANATOMY Atria: The Receiving Chambers • Small, wrinkled, protruding appendages called auricles increase the atrial volume • Internally: – Posterior portion smoothwalled – Anterior portion the walls are ridged with bundles of muscle tissue (pectinate muscles) – Anterior and posterior regions are separated by a ridge called the crista terminalis – Interatrial septum bears a shallow depression (fossa ovalis), that marks the spot where an opening, the foramen ovale, existed in the fetal heart INTERNAL HEART ANATOMY Atria: The Receiving Chambers Atria: The Receiving Chambers • • • Receiving chambers for blood returning to the heart from the circulation Small, thin-walled chambers which contract only minimally to push blood “next door” into the ventricles Blood enters the right atrium via three veins: – – – • Superior vena cava returns blood from body regions superior to the diaphragm Inferior vena cava returns blood from body areas below the diaphragm Coronary sinus collects blood draining from myocardium Blood enters the left atrium via four veins: – Pulmonary veins transport blood from the lungs back to the heart Atria: The Receiving Chambers Ventricles: The Discharging Chambers • • • • • • Together the ventricles make up most of the volume of the heart Marking the internal walls of the ventricular chambers are irregular ridges of muscle called trabeculae carneae which add support and strength Papillary muscles play a role in valve function Discharging chambers Pumps of the heart When ventricles contract, blood is propelled out of the heart into circulation: – – The right ventricle pumps blood into the pulmonary trunk, which routes the blood to the lungs where gas exchange occurs The left ventricle pumps blood into the aorta, the largest artery in the body, to the systemic trunk Ventricles: The Discharging Chambers INTERNAL HEART ANATOMY Pathway of Blood Through the Heart • The right side of the heart pumps blood into the pulmonary circuit: – Blood returning from the body is relatively oxygen-poor and carbon dioxide-rich – Blood enters the right atrium and passes into the right ventricle, which pumps it to the lungs via the pulmonary arteries (conduct blood away from the heart) – In the lungs, the blood unloads carbon dioxide and picks up oxygen (oxygenated) • The left side of the heart pumps blood into the systemic circuit Pathway of Blood Through the Heart • • • • • Freshly oxygenated blood from the lungs is carried by the pulmonary veins (toward the heart) back to the left side of the heart Left side of the heart is the systemic circuit Freshly oxygenated blood leaving the lungs is returned to the left atrium and passes into the left ventricle, which pumps it into the aorta The aorta transports blood via smaller arteries to the body tissues, where gases and nutrients are exchanged across the capillary walls Then the blood, once again loaded with carbon dioxide and depleted of oxygen, returns through the systemic veins to the right atrium via the superior vena cava and inferior vena cava Pathway of Blood Through the Heart • Although equal volumes of blood are pumped to the pulmonary and systemic circuits at any moment, the two ventricles have unequal work-loads: – Pulmonary circuit, served by the right ventricle, is a short, low-pressure circulation – Systemic circuit, associated with the left ventricle, takes a pathway through the entire body and encounters about five times as much friction, or resistance to blood flow Ventricles: The Discharging Chambers SYSTEMIC AND PULMONARY CIRCULATION Pathway of Blood Through the Heart • • • • Functional differences of the two ventricles are revealed in their anatomy The walls of the left ventricle are three-four times as thick as those of the right ventricle, and its cavity is nearly circular The right ventricular cavity is flattened into a crescent shape that partially encloses the left ventricle, much the way a hand might loosely grasp a clenched fist Consequently, the left ventricle can generate much more pressure than the right and is a far more powerful pump SYSTEMIC AND PULMONARY CIRCULATION Coronary Circulation • The heart receives no nourishment from the blood as it passes through the chamber: – The myocardium is too thick to make diffusion a practical means of nutrient delivery • The coronary circulation provides the blood supply for the heart cells: – The arterial supply of the coronary circulation is provided by the right and left coronary arteries, both arising from the base of the aorta and encircling the heart in the atrioventricular groove Coronary Circulation • The left coronary artery runs toward the left side of the heart and then divides into its major branches: – Anterior interventricular artery : follows the anterior interventricular sulcus and supplies blood to the interventricular septum and anterior walls of both ventricles – Circumflex artery: supplies the left atrium and the posterior walls of the left ventricle Coronary Circulation • The right coronary artery: courses to the right side of the heart, where it also divides into two branches – Marginal artery: serves the myocardium of the lateral right side of the heart – Posterior interventricular artery: runs to the heart apex and supplies the posterior ventricular walls • Near the apex of the heart, this artery merges (anastomoses) with the anterior interventricular artery • Together the branches of the right coronary artery supply the right atrium and nearly all the right ventricle CORONARY CIRCULATION CORONARY CIRCULATION • The arterial supply of the heart varies considerably – Example: • 15% of people, the left coronary artery gives rise to both the anterior and posterior interventricular arteries • 4% of people, a single coronary artery supplies the whole heart – There may be both right and left marginal arteries – There are many anastomoses among the coronary arterial branches: • These fusing networks provide additional (collateral) routes for blood delivery to the heart – Explains how the heart can receive adequate nutrition even when one of its coronary arteries is almost entirely occluded – Even so, complete blockage of a coronary artery leads to tissue death and heart attack CORONARY CIRCULATION • After passing through then capillary beds of the myocardium, the venous blood is collected by the cardiac veins, whose paths roughly follow those of the coronary arteries • These veins join together to form an enlarged vessel called the coronary sinus, which empties the blood into the right atrium • Obvious on the posterior aspect of the heart CORONARY CIRCULATION • The sinus has three large tributaries: – Great cardiac vein: in the anterior interventricular sulcus – Middle cardiac vein: in the posterior interventricular sulcus – Small cardiac vein: running along the heart’s right inferior margin – Additionally, several anterior cardiac veins empty directly into the right atrium anteriorly HOMEOSTATIC IMBALANCE • Blockage of the coronary arterial circulation can be serious and sometimes fatal • Angina pectoris: – Thoracic pain caused by a fleeting deficiency in blood delivery to the myocardium • May result from stress-induced spasms of the coronary arteries or from increased physical demands on the heart • Myocardial cells are weakened by the temporary lack of oxygen but do not die • Myocardial infarction (MI): – There is prolonged coronary blockage that leads to cell death – Commonly called a heart attack or coronary – Because adult cardiac muscle is essentially amitotic, most areas of cell death are repaired with noncontractile scar tissue • Whether or not a person survives a myocardial infraction depends on the extent and location of the damage • Damage to the left ventricle, which is the systemic pump, is most serious Heart Valves • Blood flows through the heart in one direction: from atria to ventricles and out the great arteries leaving the superior aspect of the heart • This one-way traffic is enforced by valves that open and close in response to differences in blood pressure on their two side Heart Valves Heart Valves Heart Valves • Two atrioventricular (AV) valves, one located at each atrial-ventricular junction ( tricuspid and bicuspid valves) prevent backflow into the atria when the ventricles contract – Right AV valve (tricuspid) has three flexible cusps (flaps of endocardium reinforced by connective tissue cores) – Left AV valve (bicuspid) has two flexible cusps • Commonly called the mitral valve because of its resemblance to the two-sided bishop’s miter or hat Heart Valves Heart Valves INTERNAL HEART ANATOMY Heart Valves • Attached to each AV valve flap are tiny white collagen cords called chordae tendineae (heart strings): – Anchor the cusps to the papillary muscles protruding from the ventricular walls HEART VALVES Heart Valves • Blood returning to the heart fills atria, putting pressure against AV valve – AV valve opens • • When the heart is relaxed, the AV valves are open hanging limply into the ventricular chambers below and blood flows into the atria and then through the open AV valves into the ventricles When ventricles contracts, compressing the blood in their chambers, the intraventricular pressure rises, forcing the blood superiorly against the valve flaps – As a result, the flaps edges meet, closing the AV valve Heart Valves • The chordae tendineae and the papillary muscles serve as guywires to anchor the valve flaps in their closed position – If the cusps were not anchored in this manner, they would be blown upward into the atria, in the same way an umbrella is blown inside out by a gusty wind Heart Valves Heart Valves • • • The aortic and pulmonary semilunar valves are found in the major arteries leaving the heart (aorta and pulmonary trunk) – They prevent backflow of blood into the ventricles Each SL valve is fashioned from three pocketlike cusps, each shaped like a crescent moon (half-moon) Mechanism of action differs from that of the AV valves – When the ventricles contracts and intraventricular pressure rises above the pressure in the aorta and pulmonary trunk, the SL valves are forced open and their cusps flatten against the arterial walls as blood rushes past them the semilunar valves are open HEART VALVE OPERATION Heart Valves • When the ventricles relax, and the blood (no longer propelled forward by the pressure of ventricular contraction) flows backward toward the heart, it fills the cusps and closes the valves HEART VALVE OPERATION Heart Valves • There are no valves guarding the entrances of the venae cavae and pulmonary veins into the right and left atria, respectively • Small amounts of blood do spurt back into these vessels during atrial contraction, but backflow is minimal because as it contracts, the atrial myocardium compresses ( and collapses) these venous entry points HOMEOSTATIC IMBALANCE • • Heart valves are simply devices, and the heart—like any mechanical pump—can function with “leaky” valves as long as the impairment is not too great Severe valve deformities can seriously hamper cardiac function – Incompetent valve: • Forces the heart to repump the same blood over and over because the valve does not close properly and blood backflows – Valvular stenosis (narrowing): • The valve flaps become stiff (typically because of scar tissue formation following endocarditis or calcium salt deposit) and constrict then opening • This stiffness compels the heart to contract more forcibly than normal • Heart’s work load increases • Heart may be severely weakened • Valve replacement: – Synthetic valve • Pig heart valve chemically treated to prevent rejection • Cryopreserved valves from human cadavers • Tissue-engineered polymer valves CARDIAC MUSCLE CELLS • Cardiac muscle (like skeletal muscle) is striated and contraction occurs via the sliding filament mechanism • In contrast to skeletal muscle, cardiac muscle is short, fat, branched • Intercellular spaces are filled with a loose connective tissue matrix containing numerous capillaries CARDIAC MUSCLE CELLS • Plasma membranes of adjacent cardiac cells interlock like the ribs of two sheets of corrugated cardboard (intercalated discs) – Disc contain anchoring desmosomes and gap junctions: • Desmosomes prevent adjacent cells from separating during contraction • Gap junctions allow ions to pass from cell to cell • Large mitochondria account for about 25% of the volume of the cardiac cell (compared with only about 2% in skeletal muscle) – Gives cardiac cells a high resistance to fatigue Mechanism and Events of Contraction • 1. Means of stimulation: • Some cardiac muscle cells are self-excitable and can initiate their own depolarization in a spontaneous and rhythmic way • 2. Organ versus motor unit contraction – Skeletal muscle: • All cells of a given motor unit (but not the entire muscle) are stimulated and contract at the same time • Impulses do not spread from cell to cell – Cardiac muscle: • The heart contracts as unit or not at all • Transmission of the depolarization wave across the heart from cell to cell via ion passage through gap junctions, which tie all cardiac muscle cells together into a single contractile unit Mechanism and Events of Contraction • 3. Length of absolute refractory period: – Refractory period: repolarization period in which the cell cannot be stimulated again until repolarization is complete • Repolarization: movement of the membrane potential to the initial resting (polarized) state – The heart’s absolute refractory period (the inexcitable period when Na+ channels are still open or are closed or inactivated) is longer than a skeletal muscle’s preventing tetanic contractions (smooth, continues contraction without any evidence of relaxation) • Long cardiac refractory period normally prevents tetanic contractions, which would stop the heart’s pumping action – 250ms in cardiac muscle (nearly as long as the contraction) – 1-2 ms in skeletal muscle (contraction last 20 to 100ms) Membrane Potential and Membrane Permeability during Action Potentials of Contractile Cardiac Muscle Cells • (a) relationship between the action potential, period of contraction, and absolute refractory period in a single ventricular cell Membrane Potential and Membrane Permeability during Action Potentials of Contractile Cardiac Muscle Cells • (b) Membrane permeability changes during the action potential (The Na+ permeability rises to a point off the scale during the action potential) Membrane Potential and Membrane Permeability during Action Potentials of Contractile Cardiac Muscle Cells • Influx of Na+ from the extracellular fluid into cardiac cells initiates a positive feedback cycle that causes the rising phase of the action potential (-90 mV to nearly + 30 mV) by opening voltage-regulated fast Na+ channels • Period of increased Na+ permeability is very brief, because the sodium gates are quickly inactivated and the Na+ channels close Membrane Potential and Membrane Permeability during Action Potentials of Contractile Cardiac Muscle Cells • Transmission of the depolarization causes causes the sarcoplasmic reticulum to release Ca2+ into the sarcoplasm (cytoplasm) • Ca2+ provides the signal for cross bridge activation Membrane Potential and Membrane Permeability during Action Potentials of Contractile Cardiac Muscle Cells • • • • Although Na+ permeability has plummeted to its resting levels and repolarization has begun by this point, the calcium surge across the membrane prolongs the depolarization potential tracing (a) At the same time, K+ permeability decreases, which also prolongs the plateau and prevents rapid repolarization As long as Ca2+ is entering, the cells continue to contract Notice in (a) that muscle tension develops during the plateau, and peaks just after the plateau ends Membrane Potential and Membrane Permeability during Action Potentials of Contractile Cardiac Muscle Cells • • • After about 200ms, the slope of the action potential tracing falls rapidly This results from closure of Ca2+ channels, Ca2+ transport from the cytosol into the extracellular space or SR (or mitochondria), and opening of voltageregulated K+ channels, which allows a rapid loss of potassium from the cell that restores the resting membrane potential During repolarization, Ca2+ is pumped back into the SR and the extracellular space Energy Requirements • Cardiac muscle has more mitochondria than skeletal muscle does, reflecting its greater dependence on oxygen for its energy metabolism – The heart relies exclusively on aerobic respiration for its energy demands (skeletal muscle during oxygen deficits can carry out anaerobic respiration) – Cardiac muscle cannot incur much of an oxygen debt and still operate effectively • Cardiac muscle is capable of switching nutrient pathways to use whatever nutrient supply is available – Thus, the real danger of an inadequate blood supply to the myocardium is lack of oxygen, not of nutrient fuels HOMEOSTATIC IMBALANCE • When a region of heart muscle is deprived of blood (is Ischemic), the oxygen-starved cells begin to metabolize anaerobically, producing lactic acid • The rising H+ level that results hinders the cardiac cells’ ability to produce the ATP they need to pump Ca2+ into the extracellular fluid • The resulting increase in intracellular H+ and Ca2+ levels causes the gap junctions (which are usually open) to close, electrically isolating the damaged cells and forcing generated action potentials to find alternate routes to the cardiac cells beyond them • If the ischemic area is large, the pumping activity of the heart as a whole may be severely impaired, leading to a heart attack HEART PHYSIOLOGY • Electrical Events: – Intrinsic conduction system is made up of specialized cardiac cells that initiate and distribute impulses, ensuring that the heart depolarizes in an orderly fashion • Even if all nerve connections to the heart are severed, the heart continues to best HEART PHYSIOLOGY • • • The autorhythmic cells have an unstable resting potential, called pacemaker potentials, that continuously depolarizes The mechanism of the pacemaker potential is believed to result from gradually reduced membrane permeability to K+ Then, because Na+ permeability is unchanged and Na+ continues to diffuse into the cell at a slow rate, the balance between K+ loss and Na+ entry is upset and the membrane interior becomes less and less negative (more positive) HEART PHYSIOLOGY • • • Ultimately, at threshold (approximately -40 mV), fast Ca2+ channels open, allowing explosive entry of Ca2+ (as well as some Na+) from the extracellular space Thus, in autorhythmic cells, it is the influx of Ca2+ (rather than Na+) that produces the rising phase of the action potential and reverses the membrane potential Once repolarization is complete, K+ channels are inactivated, K+ permeability declines, and the slow depolarization to threshold begins again Pacemaker and Action Potentials of Autorhythmic Cells of the Heart HEART PHYSIOLOGY • Autorhythmic cardiac cells are found in the following areas: – – – – Sinoatrial node Atrioventricular node Atrioventricular bundle Right and left bundle branches – Ventricular walls (Purkinje fibers) HEART PHYSIOLOGY • Impulses pass across the heart in the same order: sinoatrial node, atrioventricular node, atrioventricular bundle, right and left bundle branches, and Purkinjie fibers The intrinsic conduction system of the heart and succession of the action potential through selected areas of the heart during one heartbeat Sequence of Excitation • Sinoatrial node (SA): Pacemaker: – Located in the right atrium wall, just inferior to the entrance of the superior vena cava – Typically generates impulses about 75 times every minute • Its inherent rate in the absence of extrinsic neural and hormonal factors is closer to 100 times per minute • Sets the pace for the heart • Its characteristic rhythm (sinus rhythm) determines heart rate Sequence of Excitation • Atrioventricular node: – From the SA node, the depolarization wave spreads via gap junctions throughout the atria and via the internodal pathway to the atrioventricular (AV) node, located in the inferior portion of the interatrial septum immediately above the tricuspid valve Sequence of Excitation • Atrioventricular bundle: – From the AV node, the impulse sweeps to the atrioventricular bundle (bundle of His) in the superior oart of the interventricular septum – Although the atria and ventricles abut each other, they are not connected by gap junctions – The AV bundle is the only electrical connection between them – The balance of the AV junction is insulated by the nonconducting fibrous skeleton of the heart Sequence of Excitation • Right and Left Bundle Branches: • The AV bundle persists only briefly before splitting into two pathways—the right and left bundle branches which course along the interventricular septum toward the heart apex Sequence of Excitation • Purkinje Fibers: – Long strands of barrel-shaped cells with few myofibrils – Complete the pathway through the interventricular septum, penetrate into the apex, and then turn superiorly into the ventricular walls – Bulk of ventricular depolarization depends on these fibers and, ultimately, on cell-to-cell transmission of the impulses via gap junctions between the ventricular muscle cells – Because the left ventricle is much larger than the right, the fibers are more elaborate in the left ventricle – Directly supply the papillary muscles which are excited to contract before the rest of the ventricular muscles Sequence of Excitation • The total time between initiation of an impulse by the SA node and depolarization of the last of the ventricular muscle cells is approximately 0.22 s (220 ms) in a healthy human heart • A wringing contraction begins at the heart apex and moves toward the atria, following the direction of the excitation wave through the ventricle walls • This ejects some of the contained blood superiorly into the large arteries leaving the ventricles HOMEOSTATIC IMBALANCE • Arrhythmias: – Uncoordinated atria and ventricular contractions • Fibrillation: – Condition of rapid and irregular or out-of-phase contractions in which control of heart rhythm is taken away from the SA node by rapid activity in other heart regions • Compared to a squirming bag of worms • Fibrillating ventricles are useless as pumps • Unless defibrillated, circulation stops and brain death occurs – Accomplished by electrically shocking the heart » Depolarizes the entire myocardium (hope is: the slate is wiped clean) » SA node will begin to function normally and sinus rhythm reestablished – Implantable cardioverter defibrillators (ICDs) » Slows an abnormally fast heart or emits an electrical shock if the heart begins to fibrillate Defibrillator Defibrillator Defibrillator Defibrillator HOMEOSTATIC IMBALANCE • A small region of the heart becomes hyperexcitable, sometimes as a result of too much caffeine or nicotine, and generates impulses more quickly than the SA node – Leads to premature contractions (extrasystole) • Heart Block: – A blockage that interferes with the impulse transmission route from atria to ventricle – Interferes with the ability of the ventricles to receive pacing impulses – In total heart block no impulses get through and the ventricles beat at their intrinsic rate, which is too slow to maintain adequate circulation – A fixed-rate artificial pacemaker, set to deliver impulses at a constant rate, is usually implanted – Those suffering from partial block, in which some of the atrial impulses reach the ventricles, commonly receive demand-type pacemakers, which deliver impulses only when the heart is not transmitting on its own PACEMAKERS PACEMAKERS PACEMAKER PACEMAKER Extrinsic Innervation of the Heart • Although the basic heart rate is set by the intrinsic conduction system, fibers of the autonomic nervous system modify the beat and introduce a subtle variability from one beat to the next • The autonomic nervous system modifies the heartbeat: – Sympathetic center increases rate and depth of the heartbeat – Parasympathetic center slows the heartbeat Extrinsic Innervation of the Heart • • • Cardiac centers are located in the medulla oblongata Sympathetic (Cardioacceleratory Center) projects to motor neurons in the T1-T5 level of the spinal cord – Innervate with the SA and AV nodes, heart muscle, and the coronary arteries – Stimulates heart rate Parasympathetic (Cardioinhibitory Center) sends impulses to the dorsal vagus nucleus in the medulla, which in turn sends inhibitory impulses to the heart via branches of the vagus nerves – Project most heavily to the SA and AV nodes – Slows heart rate Autonomic innervation of the Heart Electrocardiography • The electrical currents generated in and transmitted through the heart spread throughout the body and can be amplified with an electrocardiograph – Graphic recording of heart activity obtained is called an electrocardiogram (ECG or EKG) • An ECG is a composite of all of the APs (action potentials) generated by nodal and contractile cells at a given time and not, as sometimes assumed, a tracing of a single AP • Typically 12 leads used (positioned at various sites on the body surface) • 3 are bipolar (two poles: AC current ?) leads that measure the voltage difference either between the arms or between an arm and a leg • 9 are unipolar (one pole) leads • Together the 12 leads provide a fairly comprehensive picture of the heart’s electrical activity Electrocardiography Electrocardiography • A typical ECG has three distinguishable waves called deflection waves – Small P wave: • Lasts about 0.08 s • Results from movement of the depolarization wave from the SA node through the atria • Approximately 0.1 s after the P wave begins, the atria contract The sequence of excitation of the heart related to the deflection waves of an ECG tracing Electrocardiography • P-Q interval: – The time from the beginning of atrial excitation to the beginning of ventricular excitation – About 0.16 s – Sometimes called the P-R interval because the Q wave tends to be very small – It includes atrial depolarization (and contraction) as well as the passage of the depolarization wave through the rest of the conduction system The sequence of excitation of the heart related to the deflection waves of an ECG tracing Electrocardiography • The large QRS complex: – Results from ventricular depolarization and precedes ventricular contraction – It has a complicated shape because the paths of the depolarization waves through the ventricular walls change continuously, producing corresponding changes in current direction – Average duration is 0.08 s The sequence of excitation of the heart related to the deflection waves of an ECG tracing Electrocardiography • S-T segment: – Action potential is in its plateau phase – Entire ventricular myocardium is depolarized Electrocardiography • The T wave: – Caused by ventricular repolarization – Typically lasts about 0.16 s – Repolarization is slower than depolarization • T wave is more spread out and has a lower amplitude (height) than the QRS wave – Because atrial repolarization takes place during the period of ventricular excitation, the wave representing atrial repolarization is normally obscured by the large QRS complex being recorded at the same time Electrocardiography • Q-T interval: – Lasting about 0.8 s – Period from the beginning of ventricular depolarization through ventricular repolarization Electrocardiography Electrocardiography • In a healthy heart, the size, duration, and timing of the deflection waves tend to be consistent • Changes in the pattern or timing of the ECG may reveal a diseased or damaged heart or problems with the heart’s conduction system Electrocardiography • An enlarged R wave: hints of enlarged ventricles • Flattened T wave: indicates cardiac ischemia (deficient blood flow) • Prolonged Q-T interval: reveals are polarization abnormality that increases the risk of ventricular arrhythmias ELECTROCARDIOGRAM Normal and Abnormal ECG Tracings • a: Normal sinus rhythm • b: Junctional rhythm: – SA node nonfunctional – P waves absent – Heart rate paced by AV node at 40-60 beats/min Normal and Abnormal ECG Tracings • c: Second degree heart block: – Some P waves not conducted through AV node – More P than QRS waves • Where P waves are conducted normally, the P:QRS ratio is 1:1 • In total heart block, there is no whole number ratio between P and QRS waves, and the ventricles are no longer paced by the SA node Normal and Abnormal ECG Tracings • d: Ventricular fibrillation: – Chaotic, grossly irregular, bizarre ECG deflections – Acute heart attack and electric shock HEART SOUNDS • Normal: two sounds (lub-dub) – The basic rhythm of the heart sounds is lub-dup, pause, lub-dup, pause, and so on, with the pause indicating the quiescent period • The first heart sound, lub, corresponds to closure of the AV valves – Signifies the beginning of systole when ventricular pressure rises above atrial pressure – Tends to be louder, longer, and more resonant than the second • The second heart sound, dup, corresponds to the closure of the semilunar valves – Short, sharp sound – Beginning of ventricular diastole Summary of events occurring in the Heart during the Cardiac Cycle HEART SOUNDS • Because the mitral valve closes slightly before the tricuspid valve does, and the aortic SL valve generally snaps shut just before the pulmonary valve, it is possible to distinguish the individual valve sounds by auscultating (listening for sounds within the body) four specific regions of the thorax – Notice that these four points, while not directly superficial to the valves (because the sounds take oblique paths to reach the chest wall), do define the four corners of the normal heart – Knowing normal heart size and location is essential for recognizing an enlarged (and often diseased) heart HEART SOUNDS HOMEOSTATIC IMBALANCE • Abnormal Heart Sounds: blood flows silently as long as the flow is smooth and uninterrupted – If it strikes obstructions, its flow becomes turbulent • Heart murmurs are extraneous heart sounds due to turbulent backflow of blood through a valve that does not close tightly – Fairly common in young children and some elderly people with perfectly healthy hearts • Probably because their heart walls are relatively thin and vibrate with rushing blood • Most often, murmurs indicate valve problems – Incompetent valve: • Swishing sound is heard as the blood backflows or regurgitates through the partially open valve, after the valve has (supposedly) closed – Stenotic valve: • Valve opening is narrowed • Restricts blood flow through the valve • High pitched sound or click can be detected when the valve should be wide open during systole, but is not Mechanical Events: The Cardiac Cycle • Cardiac Cycle: – Includes all events associated with the blood flow through the heart during one complete heartbeat, that is, atrial systole and diastole followed by ventricular systole and diastole • Systole is the contractile phase of the cardiac cycle • Diastole is the relaxation phase of the cardiac cycle – Marked by a succession of pressure and blood volume changes in the heart • Cardiac Cycle: • Ventricular Filling: Mid-to-Late Diastole • Ventricular Systole • Isovolumetric Relaxation: Early Diastole (1) Ventricular Filling • • • • • Mid-to-late diastole Pressure in the heart is low Blood returning from the circulation is flowing passively through the atria and the open AV valves into the ventricles Aortic and pulmonary semilunar valves are closed 70% of ventricular filling occurs during this period – • • • The remaining 30% is delivered to the ventricles when the atria contract toward the end of this phase AV valve flaps begin toward the closed position NOW the stage is set for atrial systole Following depolarization (P wave of ECG) – The atria contract, compressing the blood in their chambers • • • Causes a rise in atria pressure, which propels residual blood out of the atria into the ventricles At this point the ventricles are in the last part of their diastole and have the maximum volume of blood Then the atria relax and the ventricles depolarize (QRS complex) Summary of events occurring in the Heart during the Cardiac Cycle (2a) Ventricular Systole • As the atria relax, the ventricles begin contracting • Ventricular pressure rises, closing the AV valves • Isovolumetric contraction phase: for a split second, the ventricles are completely closed chambers and blood volume in the chambers remains constant Summary of events occurring in the Heart during the Cardiac Cycle (2b) Ventricular Systole • Ventricular pressure continues to rise and when it finally exceeds the pressure in the large arteries issuing from the ventricles, the isovolumetric stage ends as the SL valves are forced open and blood is expelled from the ventricles into the aorta and pulmonary trunk (ventricular ejection phase) – Pressure in the aorta normally reaches about 120 mm Hg Summary of events occurring in the Heart during the Cardiac Cycle (3) Isovolumetric Relaxation • • • • • • Early diastole Brief phase following the T wave Ventricles relax Blood remaining in their chambers is no longer compressed Ventricle pressure drops Blood in the aorta and pulmonary trunk backflows toward the heart, closing the SL valves – Closure of the Aortic SL valve causes a brief rise in aortic pressure as backflowing blood rebounds off the closed valve cusps (dicrotic notch) – Ventricles are totally closed Summary of events occurring in the Heart during the Cardiac Cycle (a) An ECG tracing correlated with graphs of pressure and volume changes. Time occurrence of heart sounds is also indicated. Mechanical Events: The Cardiac Cycle • All during ventricular systole, the atria have been in diastole; they have been filling with blood and the intra-atrial pressure has been rising • When blood pressure on the atrial side of the AV valves exceeds that in the ventricles, the AV valves are forced open and ventricular filling, phase 1, begins again • Atrial pressure drops to its lowest point and ventricular pressure begins to rise, completing the cycle Summary of events occurring in the Heart during the Cardiac Cycle Mechanical Events: The Cardiac Cycle • Average heart beats: – 75 beats / minute – 4500 beats / hour – 108,000 beats / day – 39,420,000 beats / year – 709,560,000 beats / 18 years – 2,759,400,000 beats / 70 years Mechanical Events: The Cardiac Cycle • Two important points: – 1. Blood flow through the heart is controlled entirely by pressure changes – 2. Blood flows down a pressure gradient through any available opening • The pressure changes, in turn, reflect the alternating contraction and relaxation of the myocardium and cause the heart valves to open, which keeps blood flowing in the forward direction • The pulmonary circulation is a low-pressure circulation as evidenced by the much thinner myocardium of its right ventricle – Systemic aortic pressure: 120 mm Hg (systolic) and 80 mm Hg (diastolic) – Pulmonary artery pressure: 24 mm Hg (systolic) and 8 mm Hg (diastolic) CARDIAC OUTPUT (CO) – The amount of blood pumped out by each ventricle in 1 minute – Stroke Volume is defined as the volume of blood pumped out of a ventricle per beat – Calculated as the product of stroke volume (SV) and heart rate (HR) • CO = HR x SV • CO = 75 beats / min x 70 ml / beat • CO = 5250 ml / min (5.25 L / min) – The normal adult blood volume is about 5 L (1.32 gallons) • Thus, the entire blood supply passes through each side of the heart once each minute Regulation of Stroke Volume Preload • The Frank-Starling law of the heart states that the critical factor controlling stroke volume is the degree of stretch of cardiac muscle cells immediately before they contract • Stretching cardiac cells can produce dramatic increases in contractile force • The most important factor stretching cardiac muscle is the amount of blood returning to the heart and distending its ventricles (a) Preload is related to the amount of blood stretching the ventricular fibers just before systole CARDIAC OUTPUT Regulation of Stroke Volume Contractility • • • Defined as an increase in contractile strength that is independent of muscle stretch end systolic volume The more vigorous contractions are a direct consequence of Ca2+ influx into the cytoplasm from extracellular fluid and the SR (sarcoplasmic reticulum) Enhanced contractility results in ejection of more blood from the heart: – Result of increased sympathetic stimulation of the heart – Increased Ca2+ promotes more cross bridge binding (actin and myosin) and enhances ventricular contractility Regulation of Stroke Volume Contractility • Factors that increase contractility: positive inotropic: – Calcium – Hormones: • Glucagon • Thyroxine • Epinephrine – Drug: • digitalis Regulation of Stroke Volume Contractility • Factors that impair or decrease contractility: negative inotropic: – Acidosis: excess H+ – Rising extracellular K+ – Drugs: • Calcium channel blockers CARDIAC OUTPUT Regulation of Stroke Volume Afterload • • Ventricular pressure that must be overcome before blood can be ejected from the heart It is essentially the back pressure exerted on the aortic and pulmonary valves by arterial blood – 80mm Hg in the aorta – 8 mm Hg in the pulmonary artery • • Normal individual: not a major concern Individual with hypertension: it reduces the ability of the ventricles to eject blood: – More blood remains in the heart after systole, resulting in increased end systolic volume (ESV) and reduced stroke volume (b) Afterload is the pressure that the ventricles must overcome to force open the aortic and pulmonary valves Preload and Afterload influence Stroke Volume CARDIAC OUTPUT Regulation of Heart Rate • A healthy cardiovascular system, SV tends to be relatively constant • When blood volume drops or heart is weakened: – SV declines and CO is maintained by increasing HR and contractility • Sympathetic stimulation of pacemaker cells increases heart rate and contractility, while parasympathetic inhibition of cardiac pacemaker cells decreases heart rate • Epinephrine, thyroxine, and calcium influence heart rate Autonomic Nervous System Regulation • Extrinsic controls affecting heart rate • When sympathetic nervous system is activated by emotional or physical stressors, such as fright, anxiety, or exercise: – Sympathetic nerve fibers release norepinephrine at their cardiac synapses • Binds to beta adrenergic receptors in the heart • Pacemaker fires more rapidly • Heart responds by beating faster CARDIAC OUTPUT Autonomic Nervous System Regulation • Sympathetic stimulation also enhances contractility by enhancing Ca2+ entry into contractile cells Mechanism by which Norepinephrine influences Heart contractility Autonomic Nervous System Regulation • The parasympathetic division opposes sympathetic effects and effectively reduces heart rate when a stressful situation has passed – May be persistently activated in certain emotional conditions, such as grief and severe depression – Responses are mediated by acetylcholine, which hyperpolarizes the membranes of its effector cells by opening K+ channels Autonomic Nervous System Regulation • Under resting conditions, both autonomic divisions continuously send impulses to the SA node of the heart, but the dominant influence is inhibitory – Exhibits vagal tone • Heart rate is generally slower than it would be if the vagal nerves were not innervating it – Cutting the vagal nerves results in an almost immediate increase in heart rate of about 25 beats / min Chemical Regulation • Hormones: – Epinephrine: liberated by the adrenal medulla during sympathetic nervous system activation • Produces the same effect as does norepinephrine released by the sympathetic nerves • Enhances heart rate and contractility – Thyroxine: thyroid gland hormone that increases metabolic rate and body heat production • Causes a slower but more sustained increase in heart rate than that caused by epinephrine Chemical Regulation • Ions: – Physiological relationships between intracellular and extracellular ions must be maintained for normal heart function – Plasma electrolyte imbalance pose real dangers to the heart HOMEOSTATIC IMBALANCE • Hypocalcemia: reduced Ca2+ blood levels – Depress the heart • Hypercalcemia: above-normal levels of Ca2+ – Prolong the plateau phase of action potential – Dramatically increase heart irritability – Lead to spastic (abnormal muscle contraction) heart contractions that permit little rest • Excess Na+ and K+ are equally dangerous – Hypernatremia : excessive Na+ • Inhibits transport of Ca2+ into the cardiac cells, thus blocking heart contraction – Hyperkalalemia: excessive K+ • Interferes with depolarization by lowering the resting potential, and may lead to heart block and cardiac arrest – Hypokalemia: low K+ • Is also life threatening, in that the heart beats feebly and arrhythmically HOMEOSTATIC IMBALANCE • Age: – Fetus: 140-160 beats/min – Gradually declines throughout life • Gender: – Females: 72-80 beats/min – Male: 64-72 beats/min • Exercise: – Raises HR by acting through the sympathetic nervous system – Increases systemic blood pressure and routes more blood to the working muscles – Trained athletes HR may be as slow as 40 beats/min • Body Temperature: – Increases HR by enhancing the metabolic rate of cardiac muscle – Exercising muscle generate heat: increase HR – Cold: decreases HR HOMEOSTATIC IMBALANCE • Tachycardia: abnormally fast heart rate (more than 100 beats/min) – Occasionally promotes fibrillation (irregular electrical activity in the heart) – Considered pathological (due to a disease) • Bradycardia: heart rate slower than 60 beats/min – Desirable, consequence of endurance training – BUT, persistent in poorly conditioned people may result in grossly inadequate blood circulation to body tissues • Frequent warning of brain edema (excessive amount of fluid) after head trauma Homeostatic Imbalance of Cardiac Output • The heart’s pumping action ordinarily maintains a balance between cardiac output and venous return • Cogestive Heart Failure: – Occurs when the pumping efficiency of the heart is so low that blood circulation cannot meet tissue needs – Reflects weakening of the myocardium by various conditions which damage it in different ways: • • • • 1. Coronary Atherosclerosis 2. Persistent High Blood Pressure 3. Multiple Myocardial Infarcts 4. Dilated Cardiomyopathy (DCM) (1) Coronary Atherosclerosis • Clogging of the coronary vessels with fatty buildup • Heart becomes increasingly hypoxic (inadequate oxygen) and begins to contract ineffectively (2) Persistent High Blood Pressure • Aortic pressure is normally 80mm Hg during diastole • When aortic diastolic blood pressure rises to 90 mm Hg or more, the myocardium must exert more force to open the aortic valve and pump out the same amount of blood • Myocardium hypertrophies (increased size of tissue / organ) • Stress takes its toll and the myocardium becomes progressively weaker (3) Multiple Myocardial Infarcts • Infarct: region of dead, deteriorating tissue resulting from a lack of blood supply • Succession of MIs depress pumping efficiency because the dead heart cells are replaced by noncontractile fibrous (scar) tissue (4) Dilated Cardiomyopathy (DCM) • Ventricles stretch and become flabby and the myocardium deteriorates • Cause often unknown • Drug toxicity (alcohol, cocaine, excess catecholamines, chemotherapeutic agents), hypothyroidism, and inflammation of the heart are implicated in some cases, as is congestive heart failure • The heart’s attempts to work harder result in increasing levels of Ca2+ in the cardiac cells which activates a calcium-sensitive enzyme that initiates a cascade which switches on genes that cause heart enlargement • Because ventricular contractility is impaired, CO is poor and the condition progressively worsens Pulmonary Congestion • Pulmonary congestion occurs when the left side of the heart fails, resulting in pulmonary edema: – Right side of the heart continues to pump blood to the lungs – Left side does not adequately eject the returning blood into the systemic circulation – Blood vessels in the lungs become engorged with blood, pressure in them increases, and fluid leaks from the circulation into the lung tissue, causing pulmonary edema Peripheral Congestion • If the right side of the heart fails, peripheral congestion occurs • Blood stagnates in body organs, and pooled fluids in the tissue spaces impair the ability of body cells to obtain adequate amounts of nutrients and oxygen and to rid themselves of wastes • Resulting edema is most noticeable in the extremities (feet, ankles, and fingers) DEVELOPMENTAL ASPECTS OF THE HEART • Embryological Development: – The heart begins as a pair of endothelial tubes that fuse to make a single heart tube with four bulges representing the four chambers – The foramen ovale is an opening in the interatrial septum that allows blood returning to the pulmonary circuit to be directed into the atrium of the systemic circuit (bypass the pulmonary circuit and the collapsed, nonfunctional fetal lungs) – The ductus arteriosus is a vessel extending between the pulmonary trunk to the aortic arch that allows blood in the pulmonary trunk to be shunted to the aorta (bypass the lungs) EMBRYONIC DEVELOPMENT EXAMPLES of CONGENITAL HEART DEFECTS Purple indicates heart areas where the defects are present HEART DEVICES DEVELOPMENTAL ASPECTS OF THE HEART • Aging Aspects of the Heart – Sclerosis and thickening of the valve flaps occurs over time, in response to constant pressure of the blood against the valve flaps – Decline in cardiac reserve occurs due to a decline in efficiency of sympathetic stimulation – Fibrosis of cardiac muscle may occur in the nodes of the intrinsic conduction system, resulting in arrhythmias – Atherosclerosis is the gradual deposit of fatty plaques in the walls of the systemic vessels