Heart AnatPhys
advertisement

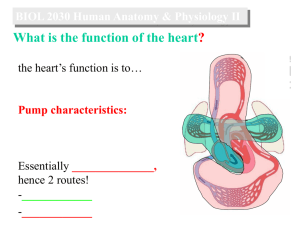
Cardiovascular System I: Heart Anatomy and Physiology Cardiovascular System Defined Gross Anatomy of the Heart Operation of Heart Valves Systemic and Coronary Circulation Cardiac Muscle Cell Anatomy and Stimulation Electrical Conduction in the Heart Cardiac Cycle and the EKG Autorhythmic Cells of the Pacemaker Stroke Volume and Contributing Factors Heart Defects The Cardiovascular System Defined What it is: • A closed system of the heart and blood vessels o The heart pumps blood o Blood vessels allow blood to circulate to all parts of the body Function: • Deliver oxygen and nutrients and to remove carbon dioxide and other waste products Heart Gross Anatomy • Location: Mediastinum of the thorax • Size: As big as your fist • Serous Membrane Coverings: 1. Epicardium (= visceral pericardium) innermost 2. Parietal pericardium outermost 3. Mediastinal pleura outside that The Heart Wall: Three Layers Visceral Parietal Myocardium Endocardium Pericardium Pericardium (Epicardium) Parietal = visceral pericardium Exception: In the heart layers, peri- is outside of epi- Coronal Section of Human Heart Flow of Blood Through the Heart Cardiovascular System I: Heart Anatomy and Physiology Cardiovascular System Defined Gross Anatomy of the Heart Operation of Heart Valves Systemic and Coronary Circulation Cardiac Muscle Cell Anatomy and Stimulation Electrical Conduction in the Heart Cardiac Cycle and the EKG Autorhythmic Cells of the Pacemaker Stroke Volume and Contributing Factors Heart Defects Operation of Heart Valves AV valves Semilunar valves Stenotic and Regurgitant Valve Conditions Heart sounds movie online Cardiovascular System I: Heart Anatomy and Physiology Cardiovascular System Defined Gross Anatomy of the Heart Operation of Heart Valves Systemic and Coronary Circulation Cardiac Muscle Cell Anatomy and Stimulation Electrical Conduction in the Heart Cardiac Cycle and the EKG Autorhythmic Cells of the Pacemaker Stroke Volume and Contributing Factors Heart Defects Coronary Circulation: Blood Feeding the Heart Superior vena cava Anastomosis (junction of vessels) Right atrium Aorta Pulmonary trunk Left atrium Left coronary artery Circumflex artery Right coronary Left artery ventricle Right ventricle Anterior Right interventricular marginal Posterior artery artery interventricular artery Posterior View of Heart Circumfle x artery Cardiovascular System I: Heart Anatomy and Physiology Cardiovascular System Defined Gross Anatomy of the Heart Operation of Heart Valves Systemic and Coronary Circulation Cardiac Muscle Cell Anatomy and Stimulation Electrical Conduction in the Heart Cardiac Cycle and the EKG Autorhythmic Cells of the Pacemaker Stroke Volume and Contributing Factors Heart Defects Homeostatic Imbalances Angina pectoris • Thoracic pain caused by a fleeting deficiency in blood delivery to the myocardium • Cells are weakened • Erythrocyte sedimentation rate (ESR) is normal in patients who experience only angina. Myocardial infarction (heart attack) • Prolonged coronary blockage due to, e.g. cardiac embolism or throbus o Causes ischemia (lack of O2) leading to muscle death • Areas of cell death are repaired with noncontractile scar tissue • ESR is elevated after an MI; can be used for diagnosis Heart Muscle, Compared Intercalated disks Cardiac Muscle Behaves as a Single Unit Nucleus Intercalated discs Cardiac muscle cell Gap junctions between cardiac cells provide electrical coupling between cells, making the myocardium behave as a single coordinated unit. Gap junctions Desmosomes In smooth and skeletal muscles, depolarization sweeps across the surface of cells. In cardiac muscle, depolarization occurs from within through gap junctions. Cardiac Cells Have Simpler T-Tubules Cardiac muscle cell Mitochondrion Intercalated disc Nucleus T tubule Mitochondrion Sarcoplasmic reticulum Z disc Nucleus Sarcolemma (b) I band A band I band Figure 18.11b Four Step Power Cycle or “Cross Bridge Cycle” Thin filament Actin Ca2+ Myosin cross bridge ADP Pi Thick filament Myosin Cross bridge formation. 1 ADP ADP Pi Pi ATP hydrolysis 2 The power (working) stroke. 4 Cocking of myosin head. ATP ATP 3 Cross bridge detachment. Figure 9.12 The Power Cycle A. Masking protein complex (tropomyosin) binds Ca++ released from the SR moves aside to expose head-binding sites B. Steps of the Power Cycle 1. "Cocked" myosin head binds to actin myofilament site 2. Head bends towards the M line (sarcomere center-line), pulling thin filament along and releasing ADP and P (broken ATP) for the power stroke 3. ATP binds to the myosin head, causing it to detach 4. Myosin head “recocks” as ATP broken down to ADP and P Longer Refractory Period in Cardiac Cells Prevents Tetanic Contractions 1 Depolarization is due to Na+ influx through fast voltage-gated Na+ channels. A positive feedback cycle rapidly opens many Na+ Long-lasting channels, reversing the tension membrane potential. inactivation ends Briefdevelopment tension developmentChannel (contraction) (contraction) this phase. Stimulus of a skeletal muscle 2 3 1 -60 Long absolute refractory period Short refractory period Time (ms) Ca+2 About 20% of the needed for the calcium pulse enters from the extracellular fluid, triggering large Ca+2 release from the SR. Tension (g) Membrane potential (mV) Action potential Plateau 2 Plateau phase is due to Ca2+ influx through slow Ca2+ channels. This keeps the cell depolarized because few K+ channels are open. 3 Repolarization is due to Ca2+ channels inactivating and K+ channels opening. This allows K+ efflux, which brings the membrane potential back to its resting voltage. Figure 18.12 Cardiac Stimulation and Contraction is Different Cell type Resting potential Stability of resting potential Source of Ca+2 for binding totroponin Main trigger of contraction cycle Range of depolarization in action potential Duration of action potential Duration of contraction Skeletal muscle -60 mV Stable, no leaks All from sarcoplasmic reticulum; released only by depolarization Neurally stimulated depolarization to single cells 100 mV (-70 mV to +30 mv) 1-2 ms; short refractory period 15-100 ms; brief contraction Cardiac muscle -80 mV Most stable but pacemaker cells drift towards 0 mV due to some open Na+ channels 10-20% extracellular Ca+2 through slow Ca+2 channels, dramatically increasing total Ca+2 release from SR Depolarization of neighboring cells 120 mV (-90 mV to +30 mV) 200+ ms; long refractory period 200+ ms; longer sustained contraction Longer Refractory Period in Cardiac Cells Prevents Tetanic Contractions Cardiovascular System I: Heart Anatomy and Physiology Cardiovascular System Defined Gross Anatomy of the Heart Operation of Heart Valves Systemic and Coronary Circulation Cardiac Muscle Cell Anatomy and Stimulation Electrical Conduction in the Heart Cardiac Cycle and the EKG Autorhythmic Cells of the Pacemaker Stroke Volume and Contributing Factors Heart Defects The Heart: Conduction System Intrinsic conduction system (nodal system) • Heart muscle cells contract, without nerve impulses, in a regular, continuous way Special tissue sets the pace 1. Sinoatrial node (pacemaker) 2. Atrioventricular node 3. Atrioventricular bundle 4. Bundle branches 5. Purkinje fibers Contraction is initiated by the sinoatrial node Sequential stimulation occurs at other autorhythmic cells Pacemaker with electrode in right ventricle Exterior conduction Homeostatic Imbalances Defects in the intrinsic conduction system may result in 1. Arrhythmias: irregular heart rhythms 2. Uncoordinated atrial and ventricular contractions 3. Fibrillation: rapid, irregular contractions; useless for pumping blood Cardiovascular System I: Heart Anatomy and Physiology Cardiovascular System Defined Gross Anatomy of the Heart Operation of Heart Valves Systemic and Coronary Circulation Cardiac Muscle Cell Anatomy and Stimulation Electrical Conduction in the Heart Cardiac Cycle and the EKG Autorhymic Cells of the Pacemaker Stroke Volume and Contributing Factors Heart Defects Filling of Heart Chambers – the Cardiac Cycle 1. Atria contract simultaneously 2. Atria relax, then ventricles contract Systole = contraction (can be subdivided into atrial and ventricular contraction but otherwise refers to ventricular systole) Diastole = relaxation (ventricular) Figure 11.6 Medulla Oblongata Cardiac Centers Accelerated and Decelerate Heart Beat The vagus nerve (parasympathetic) decreases heart rate. Dorsal motor nucleus of vagus Cardioinhibitory center Medulla oblongata Cardioacceleratory center Sympathetic trunk ganglion Thoracic spinal cord Sympathetic trunk Sympathetic cardiac nerves increase heart rate and force of contraction. AV node SA node Parasympathetic fibers Sympathetic fibers Interneurons Figure 18.15 Electrocardiograph - A chart Showing Heart Electrical Activity QRS complex Sinoatrial node Atrial depolarization Ventricular depolarization Ventricular repolarization Atrioventricular node P-Q Interval S-T Segment Q-T Interval Figure 18.16 Heart Voltages and the ECG Pressure, ECG, and Heart online EKG, Heart contractions, conduction online Ventricular systole occurs Ventricular systole occurs in T (a) Normal sinus rhythm. (b) Junctional rhythm. The SA node is nonfunctional, P waves are absent, and heart is paced by the AV node at 40 - 60 beats/min. (c) Second-degree heart block. (d) Ventricular fibrillation. These chaotic, grossly irregular ECG Some P waves are not conducted deflections are seen in acute through the AV node; hence more heart attack and electrical shock. P than QRS waves are seen. In this tracing, the ratio of P waves to QRS waves is mostly 2:1. Figure 18.18 The Heart: Cardiac Cycle lub (S1) PCG: Phonocardiograph dup (S2) Cardiovascular System I: Heart Anatomy and Physiology Cardiovascular System Defined Gross Anatomy of the Heart Operation of Heart Valves Systemic and Coronary Circulation Cardiac Muscle Cell Anatomy and Stimulation Electrical Conduction in the Heart Cardiac Cycle and the EKG Autorhythmic Cells of the Pacemaker Stroke Volume and Contributing Factors Heart Defects Cells of the S/A Pacemaker Are Autorhythmic About 1% of cardiac cells are self-excitable • Pacemaker cells have unstable resting potential due to open slow Na+ channels • As potential drifts towards neutrality, action potential initiates at threshold Ca2+ channels open • Explosive Ca+2 influx produces the rising phase of the action potential • Repolarization results from inactivation of Ca2+ channels and opening of voltage-gated K+ channels Action Potential of Autorhythmic Cells Like at the Pacemaker Threshold Action potential 2 2 3 1 1 Pacemaker potential 1 Pacemaker potential 2 Depolarization The 3 Repolarization is due to This slow depolarization is due to both opening of Na+ channels and closing of K+ channels. Notice that the membrane potential is never a flat line. action potential begins when the pacemaker potential reaches threshold. Depolarization is due to Ca2+ influx through Ca2+ channels. Ca2+ channels inactivating and K+ channels opening. This allows K+ efflux, which brings the membrane potential back to its most negative voltage. Figure 18.13 Cardiovascular System I: Heart Anatomy and Physiology Cardiovascular System Defined Gross Anatomy of the Heart Operation of Heart Valves Systemic and Coronary Circulation Cardiac Muscle Cell Anatomy and Stimulation Electrical Conduction in the Heart Cardiac Cycle and the EKG Autorhythmic Cells of the Pacemaker Stroke Volume and Contributing Factors Heart Defects Looking at Ventricular Volumes: End Diastolic Volume, End Systolic Volume, and Stroke Volume Left heart QRS P Electrocardiogram T 1st Heart sounds P 2nd Pressure (mm Hg) Dicrotic notch Aorta SV = end diastolic volume (EDV) – end systolic volume (ESV) Left ventricle Ventricular volume (ml) Atrial systole Left atrium Example: SV = 120ml/beat50ml/beat = 70 ml/beat EDV SV ESV Atrioventricular valves Aortic and pulmonary valves Phase Open Closed Open Closed Open Closed 1 2a 2b 3 1 Left atrium Right atrium Left ventricle Right ventricle Ventricular filling Atrial contraction 1 Ventricular filling (mid-to-late diastole) Isovolumetric contraction phase 2a Ventricular ejection phase 2b Ventricular systole (atria in diastole) Isovolumetric relaxation 3 Early diastole Ventricular filling Figure 18.20 Regulation of Stroke Volume Three main factors affect SV: Preload, Contractility, and Afterload 1. Preload o Degree to which the cardiac muscle cells are stretched before contraction At a certain cell length, active cross-bridges are maximized and force of contraction is maximal Cardiac cells normally shorter than optimal length Therefore, according to the Frank-Starling Law of the heart, increasing stretching (greater venous return) increases contractile force Regulation of Stroke Volume 2. Contractility • Defined as: contractile strength at a given muscle length o Contractile strength is independent of muscle stretch and EDV o Enhanced contractility causes more blood to be ejected, increasing stroke volume and decreasing ESV • Factors increasing contractility o Increased Ca2+ influx due to sympathetic stimulation o Hormones (thyroxine, glucagon, and epinephrine) o Digitalis • Factors decreasing contractility o Acidosis (H+ interferes with Ca+2 binding to myofilaments) o Increased extracellular K+ o Calcium channel blockers drugs (CCBs) like amlodipine Regulation of Stroke Volume 3. Afterload Defined as: pressure that must be overcome for ventricles to eject blood • Same as “back pressure” exerted on aortic and pulmonary valves Factor increasing afterload • Hypertension increases afterload • More blood remains in heart after systole, increasing ESV and decreasing stroke volume Heart Rate Affected by Sympathetic and Parasympathetic Systems Heart Rate increased by sympathetic nervous system • Activated by emotional or physical stressors • Activated by increased venous return (the atrial reflex) as SA node stimulated by stretch receptors • Epinephrine from adrenal medulla enhances heart rate and contractility • Thyroxine increases heart rate and enhances the effects of norepinephrine and epinephrine Heart rate decreased by parasympathetic nervous system opposes sympathetic effects Other factors include age, gender, exercise, body temperature Cardiovascular System I: Heart Anatomy and Physiology Cardiovascular System Defined Gross Anatomy of the Heart Operation of Heart Valves Systemic and Coronary Circulation Cardiac Muscle Cell Anatomy and Stimulation Electrical Conduction in the Heart Cardiac Cycle and the EKG Autorhythmic Cells of the Pacemaker Stroke Volume and Contributing Factors Heart Problems and Defects Homeostatic Imbalances Tachycardia: abnormally fast heart rate (>100 bpm) • If persistent, may lead to fibrillation Bradycardia: heart rate slower than 60 bpm • May result in grossly inadequate blood circulation • May be desirable result of endurance training Ductus Arteriosus and Foramen Ovale of the Fetus Handling of collapsed lungs Modif 1 Modif 2 Developmental Aspects of the Heart Congenital heart defects • Lead to mixing of systemic and pulmonary blood • Involve narrowed valves or vessels that increase the workload on the heart Narrowed aorta “Stenotic” Occurs in about 1 in every 500 births (a)Ventricular septal defect. (b) Coarctation of the aorta. (c) Tetralogy of Fallot. Multiple defects Age-Related Changes Affecting the Heart Sclerosis and thickening of valve flaps Decline in cardiac reserve Fibrosis of cardiac muscle Atherosclerosis Congestive Heart Failure (CHF) Progressive condition where the CO is so low that blood circulation is inadequate to meet tissue needs Caused by • Coronary atherosclerosis • Persistent high blood pressure • Multiple myocardial infarcts • Dilated cardiomyopathy (DCM) Cardiovascular System I: Heart Anatomy and Physiology Cardiovascular System Defined Gross Anatomy of the Heart Operation of Heart Valves Systemic and Coronary Circulation Cardiac Muscle Cell Anatomy and Stimulation Electrical Conduction in the Heart Cardiac Cycle and the EKG Autorhythmic Cells of the Pacemaker Stroke Volume and Contributing Factors Heart Defects