The Particle and Kinetic Theory of Matter
advertisement
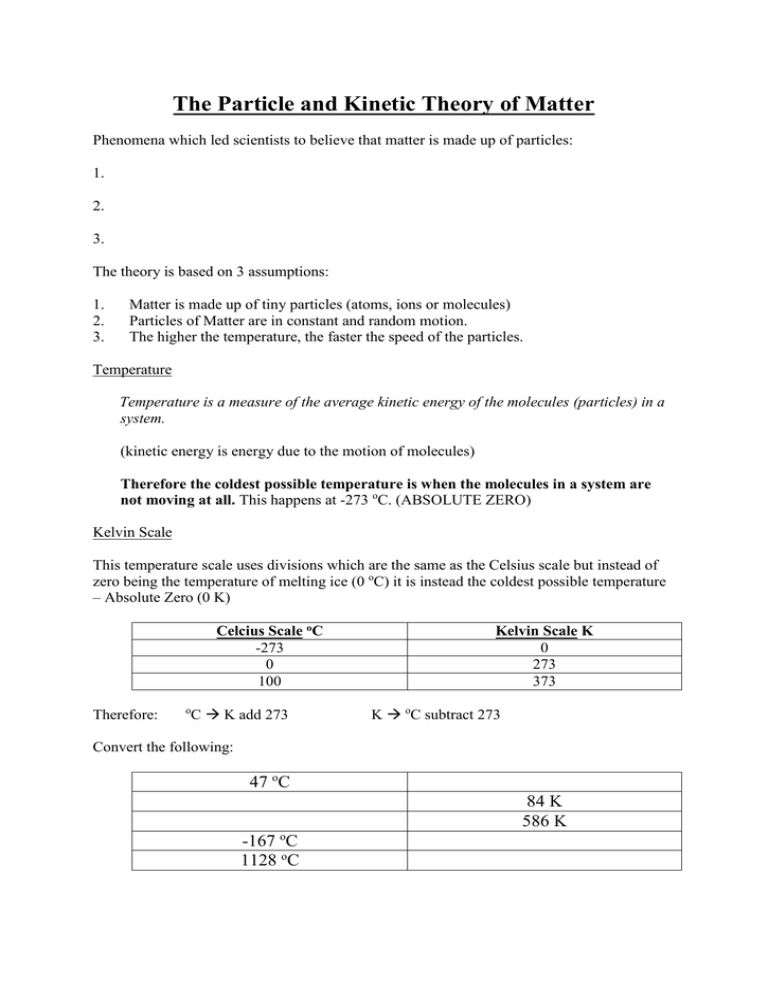
The Particle and Kinetic Theory of Matter Phenomena which led scientists to believe that matter is made up of particles: 1. 2. 3. The theory is based on 3 assumptions: 1. 2. 3. Matter is made up of tiny particles (atoms, ions or molecules) Particles of Matter are in constant and random motion. The higher the temperature, the faster the speed of the particles. Temperature Temperature is a measure of the average kinetic energy of the molecules (particles) in a system. (kinetic energy is energy due to the motion of molecules) Therefore the coldest possible temperature is when the molecules in a system are not moving at all. This happens at -273 oC. (ABSOLUTE ZERO) Kelvin Scale This temperature scale uses divisions which are the same as the Celsius scale but instead of zero being the temperature of melting ice (0 oC) it is instead the coldest possible temperature – Absolute Zero (0 K) Celcius Scale oC -273 0 100 Therefore: C K add 273 o Kelvin Scale K 0 273 373 K oC subtract 273 Convert the following: 47 oC 84 K 586 K -167 oC 1128 oC PHASES OF MATTER All matter can be placed in one of three phases; solid, liquid gas. What phase are the following at room temperature? a) water b) nitrogen Phases: SOLID c) glass d) tin LIQUID GAS Flow easily to take the shape of the container Takes the volume of container Low Can be compressed into a much smaller volume Shape Fixed shape Volume Constant volume Flow easily to take the shape of the container Constant volume Density Compressibility High Cannot be compressed into a smaller volume Medium Some can be compressed by tiny amounts. The particle model of matter helps to explain many of the different properties of matter. 1. Phases of matter SOLID Arrangement of particles Forces between particles Diagram LIQUID GAS Particles are tightly packed in a fixed pattern. Particles are still in contact but are slightly further apart than in a solid and have no fixed pattern. Particles are very far apart and have no fixed pattern. Particles make tiny vibrations about a fixed position. Particles are free to slide past each other. Particles move freely throughout the container. Strong forces. Weaker forces than in a solid. Very weak forces (almost no forces at all) 2. Phase changes Condensation Boiling or evaporation When a substance is heated the particles get more energy and move faster until they have enough energy to overcome the forces holding them together and the substance then undergoes a change of phase, eg solid to liquid or liquid to gas. e.g. Melting:- When a solid is heated the molecules move faster. At the melting point the molecules break from their fixed positions and begin to slide over one another and a liquid is formed. e.g Boiling:- _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ Examples of substances which sublime are dry ice (solid carbon dioxide) and iodine. Why is dry ice so useful? ______________________________________________________ ___________________________________________________________________________ When solid iodine is heated the black solid sublimes to form a ......................................... gas. LATENT HEAT (“Hidden” heat at phase changes) When a phase change is happening (e.g melting) the temperature stays more or less constant despite the fact that energy is being added or removed from the system. This energy is called “latent heat” because it seems to disappear. This release of energy as steam condenses is why a scald from steam can cause such a terrible wound. All the energy released damages the skin and flesh below. Steam is therefore very dangerous. Be careful when lifting the lid from a pot or kettle or (especially) microwave container. THE EFFECT OF IMPURITIES ON MELTING AND BOILING POINT OF A SUBSTANCE Melting points: These DROP. E.g when salt is added to ice it melts (and its temperature drops). This is used to remove ice from roads in some countries. Boiling points: These RISE. E.g. salt water (103 oC) has a higher boiling point than fresh water Boiling points are also affected by pressure. The higher the pressure the higher the boiling point. e.g.1 Water boils at lower temperature the higher the altitude. The boiling point on top of Mount Everest is only 70 oC. e.g.2 How does a pressure cooker work? __________________________________________________________________________ __________________________________________________________________________ Evaporation This is the loss of molecules through the surface of a liquid into the gaseous phase. It is the faster moving molecules which leave a liquid. Therefore the molecules left are moving slower on average and the liquid cools. A liquid which evaporates readily is called VOLATILE. (e.g. ether) List 4 factors which increase the rate of evaporation of a liquid like water. 1. __________________________________________________________________ 2. __________________________________________________________________ 3. __________________________________________________________________ 4. __________________________________________________________________ 3. BROWNIAN MOTION Robert Brown noticed that pollen grains on water were seen to be moving in a random way when observed under a microscope. He concluded that invisible tiny particles were moving very fast and were colliding with the pollen grains causing them to move in a haphazard way. The above observation provides some evidence for the kinetic theory. Note: smoke in air produces the same effect. In it little ash particles are seen to move as the air molecules bash into them. 4. DIFFUSION This is the spreading of one substance into another. Liquids and gasses diffuse – solids do not. When a gas jar containing air (colourless) is placed on top of a gas jar containing a redbrown gas (e.g. nitrogen dioxide or bromine), the red-brown colour spreads up into the air until the gas in both jars looks the same colour. Gases diffuse faster than liquids. Explain why. ________________________________________________________________________ ________________________________________________________________________ Hot gases diffuse faster than cool ones. Explain why. ________________________________________________________________________ ________________________________________________________________________ Heavy gas molecules diffuse more slowly than light ones. Explain why. ________________________________________________________________________ ________________________________________________________________________ e.g. Hydrogen chloride (HCl) molecules diffuse slower than ammonia (NH3). HCl Ammonium chloride NH3 5. PRESSURE OF GASES The pressure of a gas results from molecules colliding with the sides of the container. Therefore the greater the number of collisions and the greater the average force of the collisions, the greater the pressure. a) Pressure and volume. If the volume of a fixed mass of gas is decreased the pressure increases. Explain why this happens in terms of collisions. ___________________________________________________________________ ___________________________________________________________________ pressure volume b) Pressure and temperature. If the temperature of a fixed mass of gas is increased then its pressure will increase. Explain why this happens in terms of collisions. ___________________________________________________________________ ___________________________________________________________________ pressure Temperature (K)