Maternal health screening
advertisement
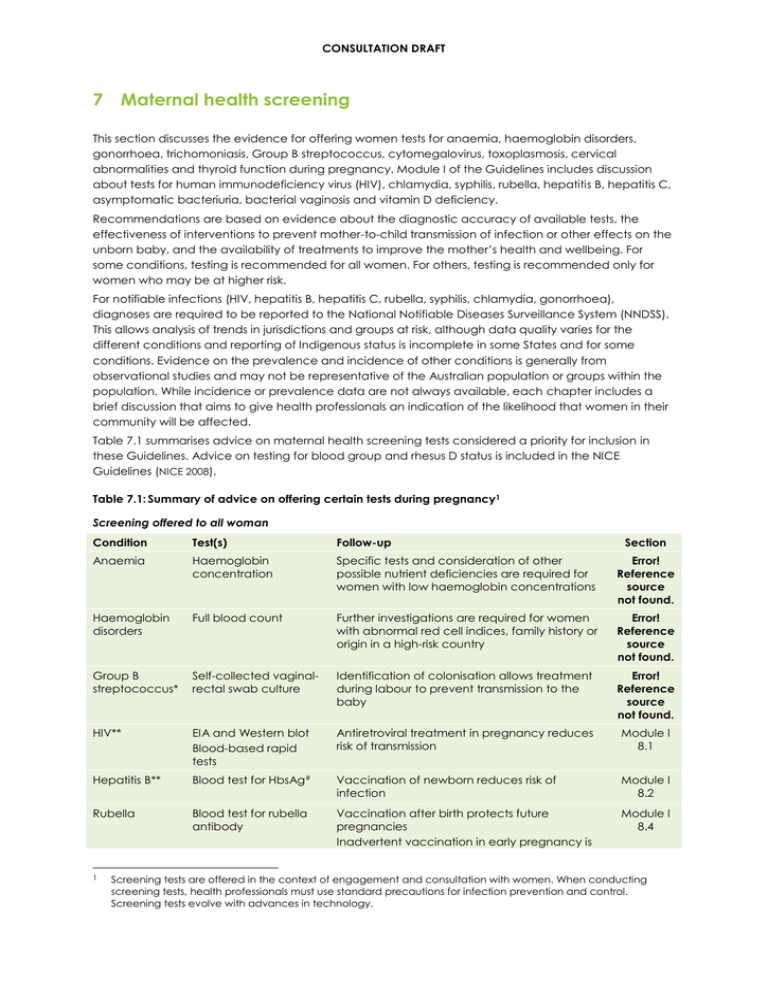
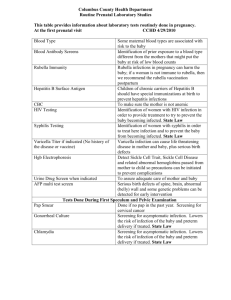
CONSULTATION DRAFT 7 Maternal health screening This section discusses the evidence for offering women tests for anaemia, haemoglobin disorders, gonorrhoea, trichomoniasis, Group B streptococcus, cytomegalovirus, toxoplasmosis, cervical abnormalities and thyroid function during pregnancy. Module I of the Guidelines includes discussion about tests for human immunodeficiency virus (HIV), chlamydia, syphilis, rubella, hepatitis B, hepatitis C, asymptomatic bacteriuria, bacterial vaginosis and vitamin D deficiency. Recommendations are based on evidence about the diagnostic accuracy of available tests, the effectiveness of interventions to prevent mother-to-child transmission of infection or other effects on the unborn baby, and the availability of treatments to improve the mother’s health and wellbeing. For some conditions, testing is recommended for all women. For others, testing is recommended only for women who may be at higher risk. For notifiable infections (HIV, hepatitis B, hepatitis C, rubella, syphilis, chlamydia, gonorrhoea), diagnoses are required to be reported to the National Notifiable Diseases Surveillance System (NNDSS). This allows analysis of trends in jurisdictions and groups at risk, although data quality varies for the different conditions and reporting of Indigenous status is incomplete in some States and for some conditions. Evidence on the prevalence and incidence of other conditions is generally from observational studies and may not be representative of the Australian population or groups within the population. While incidence or prevalence data are not always available, each chapter includes a brief discussion that aims to give health professionals an indication of the likelihood that women in their community will be affected. Table 7.1 summarises advice on maternal health screening tests considered a priority for inclusion in these Guidelines. Advice on testing for blood group and rhesus D status is included in the NICE Guidelines (NICE 2008). Table 7.1: Summary of advice on offering certain tests during pregnancy1 Screening offered to all woman Condition Test(s) Follow-up Anaemia Haemoglobin concentration Specific tests and consideration of other possible nutrient deficiencies are required for women with low haemoglobin concentrations Error! Reference source not found. Haemoglobin disorders Full blood count Further investigations are required for women with abnormal red cell indices, family history or origin in a high-risk country Error! Reference source not found. Group B streptococcus* Self-collected vaginalrectal swab culture Identification of colonisation allows treatment during labour to prevent transmission to the baby Error! Reference source not found. HIV** EIA and Western blot Blood-based rapid tests Antiretroviral treatment in pregnancy reduces risk of transmission Module I 8.1 Hepatitis B** Blood test for HbsAg# Vaccination of newborn reduces risk of infection Module I 8.2 Rubella Blood test for rubella antibody Vaccination after birth protects future pregnancies Inadvertent vaccination in early pregnancy is Module I 8.4 1 Section Screening tests are offered in the context of engagement and consultation with women. When conducting screening tests, health professionals must use standard precautions for infection prevention and control. Screening tests evolve with advances in technology. CONSULTATION DRAFT Condition Test(s) Follow-up Section highly unlikely to harm the baby Syphilis# Treponemal EIA tests Onsite tests Treatment benefits mother and prevents congenital syphilis Module I 8.6 Asymptomatic bacteriuria Midstream urine culture Treatment reduces risk of pyelonephritis Module I 8.7 Notes: * According to organisational policy. ** Specialist care and psychosocial support are required for women with HIV or hepatitis B. # Psychosocial support, partner testing and contact tracing is required for women with a sexually transmitted infection. The 2012 Australasian Society for HIV Medicine National Hepatitis B testing policy is available at http://testingportal.ashm.org.au/hbv. EIA=enzyme immunoassay; HbsAg=hepatitis B surface antigen; HIV=human immunodeficiency virus. Screening offered to woman at increased risk Condition Offer test to: Test(s) Follow-up Section Gonorrhoea* Women with known risk factors or living in areas where prevalence is high Vaginal, urine or endocervical specimens PCR Treatment may prevent neonatal infection Error! Reference source not found. Trichomoniasis* Women with symptoms Culture or PCR testing of vaginal swabs Treatment may prevent certain infections in the newborn but is associated with adverse effects Error! Reference source not found. Toxoplasmosis Women may request testing based on exposure to sources Studies into tests are limited and inconclusive Insufficient evidence on treatment. Advice on prevention may reduce the risk of infection Error! Reference source not found. Cytomegalovirus Women who have frequent contact with large numbers of very young children Studies into tests are limited and inconclusive Insufficient evidence on treatment. Advice on prevention may reduce the risk of infection Error! Reference source not found. Cervical abnormalities Women who have not had a Pap smear in the past 2 years Pap smear Allows detection of precancerous cervical abnormalities Error! Reference source not found. Thyroid function Women with symptoms or risk factors Blood test for thyroid-stimulating hormone Treatment improves obstetric outcomes Error! Reference source not found. Chlamydia* Women younger than 25 years All pregnant women in areas of high prevalence First pass urine PCR Treatment may reduce the risk of preterm birth, premature rupture of the membranes and low birth weight Module I 8.5 Hepatitis C** Women with a history of risk factors for hepatitis C Blood test for hepatitis antibody RNA if antibodies detected No interventions are currently proven to prevent transmission Module I 8.3 Asymptomatic bacterial vaginosis Women with a previous preterm birth High vaginal swab Amsel’s criteria Nugent’s criteria Early treatment (<20 wks) may reduce risk of premature rupture of the membranes and low birth weight Module I 8.8 Vitamin D Women considered Blood test for Women at high risk of Module I CONSULTATION DRAFT Condition Offer test to: Test(s) Follow-up deficiency to be at risk serum 25-OHD deficiency may benefit from supplementation Notes: Section 8.9 * Psychosocial support, partner testing and contact tracing is required for women with a sexually transmitted infection. ** Specialist care and psychosocial support are required for women with hepatitis C. 25-OHD=25-hydroxyvitamin D; PCR=polymerase chain reaction; RNA=ribonucleic acid. Considerations before testing Before tests are carried out, it is essential that: • women are informed that it is their choice to have tests; • women are able to give informed consent — verbal discussion should cover the reasons for testing, harms and benefits and associated treatments and be supported by appropriate resources (eg written materials, audio or video); • women have opportunities to ask questions about tests and treatments; • women are reassured that test results remain confidential (unless the condition is notifiable); • discussions about consent are documented by the health professional involved; • women who decline testing are offered the opportunity to discuss any concerns they may have without being coerced to reconsider the test; and • there are processes for follow-up of women with a positive test result, their babies and, in some situations, partners. Discussion of testing should be approached with sensitivity, particularly when there is a potential for testing to raise maternal anxiety or if testing is for a sexually transmitted infection. Considerations after a positive test result • Referral for specialist care: For some conditions, such as haemoglobin disorders and thyroid dysfunction, specialist involvement will be required. • Psychosocial support: Diagnosis of a condition that may affect pregnancy and/or the health of the baby can be distressing, particularly if there are no interventions that can change outcomes. Women should be given information about available supports and assisted to access these. • Sexually transmitted infections: If a sexually transmitted infection is identified, there is an increased risk of other sexually transmitted infections. Partner testing and contact tracing are also advisable. • Blood-borne infections: Specific supports are likely to be required for women identified as using intravenous drugs. • Notification: State/Territory legislation on notification of communicable diseases must be followed. Type of test The tests discussed in this section are those currently in use in Australia. With continuous advances in technology and testing, techniques change rapidly. The most appropriate test may also depend on the particular clinical setting. Testing in rural and remote areas It is acknowledged that in Australia, access to tests may vary (eg due to distance from pathology services), storing tests and samples appropriately may be challenging (eg due to high temperatures or humidity) and there may be difficulties in recalling women to receive test results. In these situations, resources should be focused on responding to local needs (eg ensuring that tests are available to identify highly prevalent conditions). Screening for diabetes during pregnancy Diabetes diagnosed during pregnancy (gestational diabetes) is a complex area and approaches to screening, diagnosis and treatment are currently being reviewed by a number of relevant organisations including the World Health Organization, Australasian Diabetes in Pregnancy Society, Royal Australian and New Zealand College of Obstetricians and Gynaecologists, the Royal Australian College of CONSULTATION DRAFT Pathologists and the New Zealand Ministry of Health. Consequently an additional review of the gestational diabetes literature was not conducted as part of the development of these Guidelines. It is anticipated that a separate evidence-based guideline on diabetes and pregnancy will be need to be prepared in the future and that a summary of relevant guidance be included in future versions of these Guidelines.