denature proteins-enzymes
advertisement

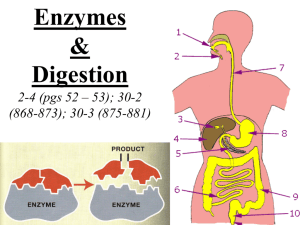
Denature proteins Enzymes & Digestion Page 48, 99-105 662-670 Protein structure (review) R groups hydrophobic interactions disulfide bridges (H & ionic bonds) 3° multiple polypeptides hydrophobic interactions 1° amino acid sequence peptide bonds determined by DNA 4° 2° R groups H bonds Primary (1°) structure • Order of amino acids in chain – amino acid sequence determined by gene (DNA) – slight change in amino acid sequence can affect protein’s structure & its function • even just one amino acid change can make all the difference! lysozyme: enzyme in tears & mucus that kills bacteria Just 1 out of 146 amino acids! Sickle cell anemia I’m hydrophilic! But I’m hydrophobic! In Biology, Protein denaturation size doesn’t matter, • Unfolding a protein SHAPE matters! – conditions that disrupt H bonds, ionic bonds, disulfide bridges • temperature • pH • salinity – alter 2° & 3° structure • alter 3-D shape – destroys functionality • some proteins can return to their functional shape after denaturation, many cannot •A.Enzymes work best within a certain environment •B. Denaturation-Enzymes can be permenantly destroyed by changing their shape! •Denaturation is caused by: • High temperatures • Acidity (pH changes) • Solvents (alcohols, like rubbing alcohol) • Other chemicals that break the bonds inside the protein that help it keep its shape What role do enzymes play in living things? some important chemical reactions are too slow or have a high activation energy (require too much energy to start the reaction) catalysts – substances that speed up the rates of chemical reactions enzymes are proteins that act as natural catalysts enzymes are very SPECIFIC, catalyzing only 1 chemical reaction. enzyme-substrate complex where reactant (substrate) meets enzyme & enough energy is provided to start the reaction substrate (reactant) binds to active site on specific enzyme (complimentary fit – like a lock & key) enzymes remain unchanged Enzyme-substrate Complex http://highered.mcgrawhill.com/sites/0072495855/student_view0/chapter2/animation__how_enzymes_work.html Examples of Enzymes in the Digestive System amylase breaks down carbohydrates (starch into disaccharides) pepsin breaks down proteins lypase breaks down fat maltase, Breaks down carbohydrates sucrase, (disaccharides into monosaccharides) lactase Regulation of Enzyme Activity enzymes can be affected by temperature and pH enzymes produced by human cells work best at normal human body temperature stomach enzyme pepsin works best in acidic conditions • •1. .Concentration of substrate: •2. Concentration of enzyme • Harder for the substrate to randomly find the active site on the enzyme •3.Temperature • At higher temperatures molecules move faster, so the substrate has a better chance of finding the active site. • Like bumpercars—more collisions when you hit the gas! Digestive System food travels through many organs of the digestive system broken down into usable nutrients mouth: 1 minute mechanical digestion via teeth chemical digestion via amylase esophagus: 2-3 seconds tube that leads to the stomach via peristalsis amylase pepsin stomach: 2-4 hours mechanical digestion via muscle churning chemical digestion via pepsin small intestine: 3-5 hours bile (made by liver & stored in gall bladder) chemically breaks down fat along with lipase enzymes maltase, sucrase, and lactase break down carbs large intestine: 10 hrs – several days absorbs H2O and eliminates wastes Lipase maltase sucrase lactase Obtaining Macromolecules thru the Food Pyramid