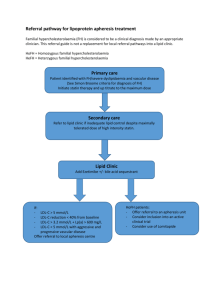
New developments in lipid management
CGR 0800 h 11 May 2015
Rob Hegele MD FRCPC FACP
Distinguished Professor of Medicine and Biochemistry
Western University
London, Canada
hegele@robarts.ca
Financial disclosure: speaker and ad board member for
Aegerion, Amgen, Merck, Pfizer, Sanofi, Valeant
Overview
existing drugs
new drugs
Overview
existing drugs
new drugs:
PCSK9 inhibitors
LDL-C and CHD risk
Lower on-Rx LDL-C and reduced risk
% incidence of events
35
Major cardiovascular events
30
Major coronary events
25
Major cerebrovascular events
20
15
10
5
0
≥4.52
3.88-<4.52 3.23-<3.88 2.58-<3.23 1.94-<2.58 1.29-<1.94
<1.29
Achieved LDL-C concentration in mmol/L
Boekholdt SM et al. JACC 2014; 64:5485-94
Reduced all-cause mortality with statins
4S Investigators Lancet 2004; 364:771-7.
Second line drugs
1. Bile acid sequestrants
2. Ezetimibe
3. Fibrates
4. Niacin
Bile acid sequestrants
Lipid Research Clinics Coronary Primary Prevention Trial (LRC-CPPT)
Life-Table Cumulative Incidence (%)
12
Life-table cumulative
incidence of primary end point
(definite CHD death and/or definite
nonfatal MI) in treatment groups,
computed by Kaplan-Meier method.
10
8
Placebo
6
4
Cholestyramine resin
2
0
1
2
3
4
5
6
Years of Follow up
7
8
9
Lipid Research Clinics. JAMA 1984;251:351-364.
8
LDL-C and Lipid Changes
1 Yr Mean
Mean LDL-C (mmol/L)
2.5
2.25
LDL-C
TC
TG
HDL
hsCRP
Simva
1.81
3.75
1.55
1.24
3.8 mg/dl
EZ/Simva
1.38
3.25
1.36
1.26
3.3 mg/dl
Δ in mmol/L
-0.43
-0.50
-0.19
+0.2
-0.5mg/dl
2.0
1.75
median time avg
1.8 vs. 1.4 mmol/L
1.5
1.25
1.0
QE R
Number at risk:
1 4 8 12 16 24 36 48 60 72 84 96
Time since randomization (months)
AHA Scientific Sessions, 17 Nov 2014
Primary Endpoint — ITT
Cardiovascular death, MI, documented unstable angina requiring
rehospitalization, coronary revascularization (≥30 days), or stroke
HR 0.936 CI (0.887, 0.988)
p=0.016
Simva — 34.7%
2742 events
NNT= 50
EZ/Simva — 32.7%
2572 events
7-year event rates
AHA Scientific Sessions, 17 Nov 2014
Primary and 3 Prespecified
Secondary Endpoints — ITT
Simva* EZ/Simva* p-value
Primary
CVD/MI/UA/Cor Revasc/CVA
Secondary #1
All D/MI/UA/Cor Revasc/CVA
Secondary #2
CHD/MI/Urgent Cor Revasc
Secondary #3
CVD/MI/UA/All Revasc/CVA
0.936
0.948
0.912
0.945
0.8
1.0
1.1
Ezetimibe/Simva
Simva
Better
Better
34.7
32.7
0.016
40.3
38.7
0.034
18.9
17.5
0.016
36.2
34.5
0.035
*7-year
event rates (%)
UA, documented unstable angina requiring rehospitalization; Cor Revasc, coronary revascularization
(≥30 days after randomization); All D, all-cause death; CHD, coronary heart disease death;
All Revasc, coronary and non-coronary revascularization (≥30 days)
AHA Scientific Sessions, 19 Nov 201
Safety — ITT
No statistically significant differences in cancer or
muscle- or gallbladder-related events
Simva
n=9077
%
EZ/Simva
n=9067
%
p
ALT and/or AST≥3x ULN
2.3
2.5
0.43
Cholecystectomy
1.5
1.5
0.96
Gallbladder-related AEs
3.5
3.1
0.10
Rhabdomyolysis*
0.2
0.1
0.37
Myopathy*
0.1
0.2
0.32
Rhabdo, myopathy, myalgia with CK elevation*
0.6
0.6
0.64
Cancer* (7-yr KM %)
10.2
10.2
0.57
* Adjudicated by Clinical Events Committee
% = n/N for the trial duration
IMPROVE-IT vs. CTT:
Ezetimibe vs. Statin Benefit
IMPROVE-IT
CTT Collaboration.
Lancet 2005; 366:1267-78;
Lancet 2010;376:1670-81.
Fibrates: Gemfibrozil Reduced MCVE
in Patients with CAD by 22%
Rubins HB et al. NEJM 1999; 341: 410-8
ACCORD-Lipid: MACE
Possible role for fibrates
High TG, low HDL-C subgroups
Normolipidemic subgroups
16
Sacks F et al. N Engl J Med 2010; 363:692-695
Coronary Drug Project:
Effect of Niacin in Post-MI Patients
Cumulative Rate of Nonfatal MI in
Post-MI Patients Treated With Niacin or Placebo
Cumulative Event Rate (%)
15
Recurrent
nonfatal MI
Placebo
Niacin
10
27%
5
(P < 0.004)
0
12
34
36
48
Months of Follow-up
60
Patients receiving niacin (n=1119) vs patients receiving placebo (n=2789). Total mortality
was similar between the 2 groups at 5 years.
The Coronary Drug Project Research Group. JAMA. 1975;231:360-381.
HPS2-THRIVE: Major Vascular Events on
Niacin/Laropiprant (ERN/LRPT)
Patients suffering events (%)
20
15
15.0%
14.5%
10
Placebo
ERN/LRPT
5
Risk ratio 0.96
(95% CI 0.90–1.03)
Logrank P=0.29
0
0
1
2
Years of follow-up
3
4
Armitage J, et al "HPS2-THRIVE: Randomized placebo-controlled trial of ER Niacin and laropriprant in 25,673 patients
with pre-existing cardiovascular disease" ACC 2013.
CVD end point reduction
Drug class
Bile acid
sequestrants
Ezetimibe
Fibrates
Niacin
No background With background
statin
statin
Yes (LRC-CPPT)
Not done
Not done
Yes (HHS, VA-HIT)
Yes (CDP)
Yes (SHARP; IMPROVE-IT)
No (ACCORD, FIELD)
No (AIM-HIGH, HPS2)
Combination treatment: safety
Very safe:
statin + bile acid sequestrant
statin + ezetimibe
Quite safe:
statin + niacin
statin + fenofibrate
statin + bezafibrate
Riskier statins: lova, simva
Reduce dose:
fenofibrate if creatinine > 150
Avoid:
statin + gemfibrozil
20
Non-pharmacological LDL-lowering
Compound
Dose
% LDL lowering
Evidence level
Isoflavones (soy protein powder)
50-100 mg
3-11%
A-I
Soluble fibre
5-15 g
5-20%
A-I
Oatmeal
60 g
2-6%
A-I
Plant sterols
1.3 g
4-13%
A-I
AHA Step 2 diet
5-10%
A-I
Mediterranean diet
5-10%
A-I
Portfolio diet
10-20%
A-I
Almonds
50-80 g
5%
B-I
Green tea extract
1.2 g
10%
B-I
High carb diet
60% of calories
5-10%
B-I
High protein diet
25% of calories
5-10%
B-I
Red yeast rice
1-2 g
7-20%
A-IIa
Guggulipid
100 mg
12%
A-IIb
Huang et al. Can J Cardiol 2011: 488-505
Looking forward to the 2015 guidelines
• keep LDL-C targets
• combination Rx
• non-statin LDL-C lowering
• non-HDL-C as alternate
• non-fasting lipids
• ongoing RCTs – PCSK9i lower LDL-C < 1.0 mmol/L
• ongoing RCTs – CETP inhibitors
Emerging lipid therapies
- lomitapide
- mipomersen
- anti-PCSK9
- CETP inh (ana, eva)
effect
lowers LDL-C by 40%
lowers LDL-C by 40%
lowers LDL-C by 60%
lowers LDL-C by 30%
- alipogene tiparvovec
- anti-APOC3
- anti-ANGPTL
lowers TG by 30%
lowers TG by 50%
lowers TG by 50%
Four Mechanisms for Reducing LDL-C
Lilly SM, Rader DJ. Curr Opin Lipid. 2007;18:650–655.; Shinkai H. Vasc Health Risk Manag. 2012;8:323-331.
Emerging lipid therapies
Emerging lipid therapies
Proprotein
Emerging lipid therapies
Proprotein
Convertase
Emerging lipid therapies
Proprotein
Convertase
Subtilisin
Emerging lipid therapies
Proprotein
Convertase
Subtilisin
Kexin
Emerging lipid therapies
Proprotein
Convertase
Subtilisin
Kexin
9
PCSK9 inhibitors
-
very potent LDL-C reduction: up to 70%
non-statin mechanism
mAbs: sc q2 or q4 wk
competitive environment
signal for 50% reduced MCVE
Loss-of-Function Mutations in PCSK9 are Associated
with Lower Serum LDL-C and Lower Incidence of CHD
No Mutation
(N=3 278)
50th Percentile
30
20
Frequency (%)
10
0
0
1.3
2.6
3.9
5.2
6.5
7.8
PCSK9142X or PCSK9679X
(N=85)
30
Coronary Heart Disease (%)
12
8
4
0
No
Yes
PCSK9142X or PCSK9679X
20
PCSK9 mutations were associated with a 28% reduction
in mean LDL-C and an 88% reduction in the lifetime risk
of CHD (P = 0.008 for the reduction; hazard ratio, 0.11;
95% CI, 0.02 to 0.81; P = 0.03)
10
0
0
1.3
2.6
3.9
5.2
6.5
7.8
Plasma LDL-C in Black Subjects (mmol/L)
Cohen JC et al. N Engl J Med. 2006;354:1264-72.
Loss-of-Function Mutations in PCSK9 are Associated
with Lower Serum LDL-C and Lower Incidence of CHD
No Mutation
(N=3 278)
50th Percentile
30
20
Frequency (%)
10
0
0
1.3
2.6
3.9
5.2
6.5
7.8
PCSK9142X or PCSK9679X
(N=85)
30
Coronary Heart Disease (%)
12
8
4
• PCSK9 LOF mutations found in 1% to 4%
of population
0
No
Yes
• Associated with
PCSK9
or PCSK9
142X
679X
Lower serum LDL-C
PCSK9 mutations were associated with a 28% reduction
10 Lower incidence of coronary heart disease
20
in mean LDL-C and an 88% reduction in the lifetime risk
of CHD (P = 0.008 for the reduction; hazard ratio, 0.11;
95% CI, 0.02 to 0.81; P = 0.03)
0
0
1.3
2.6
3.9
5.2
6.5
7.8
Plasma LDL-C in Black Subjects (mmol/L)
Cohen JC et al. N Engl J Med. 2006;354:1264-72.
Serum
LDL-Cholesterol
Bindsthe
to LDL-Receptors.
In
the
Presence
of
PCSK9,
LDL-R Is Degraded
Following Internalization, LDL is Degraded and the
and
DoesRecycled
Not Cycle Back to Cell Surface
Receptor
Qian YW, et al. J Lipid Res. 2007;48:1488-1498.
Horton JD, et al. J Lipid Res. 2009;50(suppl):S172-S177.
Blocking
PCSK9
Activity
Inhibits
Monoclonal
Antibody
binds
to PCSK9 and
Intracellular
Degradation
of LDL-R
inhibits Binding
to the LDL-Receptor
Qian YW, et al. J Lipid Res. 2007;48:1488-1498.
Horton JD, et al. J Lipid Res. 2009;50(suppl):S172-S177.
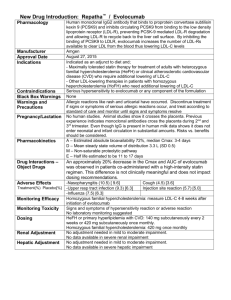
PCSK9-Directed Therapies in Development
Company
Drug
Agent
Indication
Phase
Inhibition of PCSK9 binding to LDLR
Amgen
Evolocumab
Fully Human mAb
Hypercholesterolemia
3
Sanofi/Regeneron
Alirocumab
Fully Human mAb
Hypercholesterolemia
3
Bococizumab
mAb
Hypercholesterolemia
3
Novartis
LGT209
mAb
Hypercholesterolemia
2
Roche/ Genentech
RG7652
mAb
Hypercholesterolemia
2
LY3015014
mAb
Hypercholesterolemia
2
Adnexins
Hypercholesterolemia
1
ALN-PCS02
siRNA oligonucleotides
Hypercholesterolemia
2
TBD
Antisense oligonucleotide
Hypercholesterolemia
Preclinical
SX-PCK9
Small peptide mimetic
Hypercholesterolemia
Preclinical
Shifa Biomedical
TBD
Small molecule
Metabolic Disorders
Preclinical
Cadila Healthcare
TBD
Small molecule
Pfizer/Rinat
Neuroscience
Eli-Lilly
PCSK9 protein binding fragment
BMS/ Adnexus
BMS-962476
Inhibition of PCSK9 synthesis (gene silencing)
Alnylam
Idera
Inhibition of PCSK9 autocatalytic processing
Seometrix
Preclinical
mAb: monoclonal antibody; CVD: cardiovascular disease
Adapted from Rhainds D, et al. Clin Lipidol. 2012;7:621-640.;Lambert G, et al. J Lipid Res. 2012;53:2515-24;clinicaltrials.gov; Stein EA.
Swergold GR. Curr Atheroscler Rep. 2013:15:310.
Terminology of Monoclonal Antibodies
Mouse
(% human protein) (0% human)
Source
Generic suffix:
High
-omab
Chimeric
(65% human)
Humanized
(> 90% human)
-ximab
-zumab
Potential for immunogenicity
1. Weiner LM. J Immunother. 2006;29:1-9.; 2. Yang XD, et al. Crit Rev Oncol Hematol. 2001;38:17-23.;
3. Lonberg N. Nat Biotechnol. 2005;23:1117-1125.; 4. Gerber DE. Am Fam Physician. 2008;77:311-319.
Human
(100% human)
-umab
Low
Alirocumab: Phase II/III LDL-C Lowering
Summary
Open-label
HeFH
12 wk RCT+ 52 wk open-label extension
add-on therapy,
Mean LDL-C 3.9 mmol/L
Placebo Q2W
n=15
n=45
0
Q2W
n=54
LS Mean % Change in LDL-C Level
at Week 8/12 LOCF
-10
Hypercholesterolemia
24 weeks
N=103, monotherapy
Inclusion LDL-C 2.6-4.9 mmol/L
Hypercholesterolemia
12 weeks
N=88, add-on therapy,
inclusion LDL-C ≥2.6 mmol/L
Placebo Q2W †
n=31 n=30
Q2W
n=31
150
50mg
mg 100 mg
150 mg 150 mg
ODYSSEY MONO3
Dose Ranging
extension1
Q2W
n=29
Q4W
n=28
150 mg
200 mg
Q4W
n=30
Q2W
n=52
300 mg
150 mg
EZE
n=51
10 mg
-5.0%
-10.7%
-16%
-20
-30
-40
-40.0%*
-50
-43%*
-48%*
-60
-70
-47%
-59.5%
-67.9%
† LDL-C
-64% *
-72%*
Placebo
*P<0.0001 vs. Placebo
1. Stein EA, et al. Presented at ACC 2014. Abstract 1183-126.
2. McKenney JM, et al. J Am Coll Cardiol. 2012;59(25):2344-53.
3. Roth EM, et al. Poster presentation at ACC 2014. Abstract 1183-125..
reductions with SAR236553 were
similar among atorvastatin doses (10, 20, 40 mg)
Alirocumab
Ezetimibe
Alirocumab was well tolerated with no evidence of any liver or
creatine kinase elevations. Injection site bruising the most
frequently reported adverse event.
Bococizumab: Efficacy as add-on therapy in
hypercholesterolemia 24 week study
LS Mean % Change in LDL-C Level
at Week 8/12 LOCF
Q2W
Q2W
Q2W
Q4W
0
-10
50 mg
100 mg
150 mg
150 mg
200
150 mg
mg
Q4W
300 mg
150 mg
-20
-30
-40
-50
-60
Phase 2 Study
Hypercholesterolemia
N=354, add-on therapy,
inclusion LDL-C ≥2.1 mmol/L
-28%
-34%
-45%
-45%
-53%
-70
• Incidence and profile of adverse events similar across groups.
Ballantyne CM, et al. Poster presentation at ACC 2014. Abstract 1183-129
Evolocumab: effect on LDL-C
LDL-C 3.1 mmol/L
LDL-C 1.24 mmol/L
Sabatine M et al. NEJM Mar 2015 online
Evolocumab: CVD reduction
Sabatine M et al. NEJM Mar 2015 online
Evolocumab: adverse events
Sabatine M et al. NEJM Mar 2015 online
Alirocumab: effect on LDL-C
Robinson J et al. NEJM Mar 2015 online
Alirocumab: CVD reduction
Robinson J et al. NEJM Mar 2015 online
Alirocumab: adverse events
Robinson J et al. NEJM Mar 2015 online
Four Mechanisms for Reducing LDL-C
Lilly SM, Rader DJ. Curr Opin Lipid. 2007;18:650–655.; Shinkai H. Vasc Health Risk Manag. 2012;8:323-331.
Lomitapide: LDL-C change from baseline
Mean % change in LDL-C (±95%CI)
(Week 126 Completers Population)
10
Phase 3
0
Long-Term Extension
–10
–20
–30
–40
–50
–60
–70
–80
n:
0
10
18
26
36
46
56
66
Week
78
90
102
114
126
17
17
16
17
17
17
17
17
17
17
17
17
17
48
Proprietary. ©2014 Aegerion Pharmaceuticals, Inc. All Rights Reserved.
Juxtapid is a trademark of Aegerion Pharmaceuticals, Inc. Licensed User Aegerion Pharmaceuticals (Canada) Ltd.
APOB antisense: mipomersen in HoFH
Raal D et al. Lancet 2010; 375:998-1006.
CETP inhibition: effect on LDL-C
Kastelein J et al. Lancet 2015 75:998-1006.
CETP inhibition: effect on HDL-C
Kastelein J et al. Lancet 2015 75:998-1006.
Summary
-
statins are good
LDL-C targets will remain in guidelines
second line drugs work – depends on context
novel Rx for LDL-C:
-
PCSK9 inhibitors
lomitapide
APOB antisense
CETP inhibitors