IN SITU ENVIRONMENTAL (WET CELL) TEM
advertisement

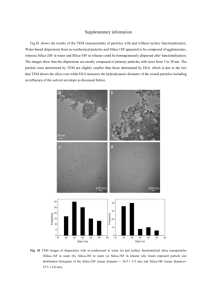
IN SITU (WET) ENVIRONMENTAL TEM Wen-An Chiou Materials Characterization Center (MC2) and Department of Chemical Engineering and Materials Science University of California at Irvine, Irvine, CA 92612-2575 Pan-American Advanced Studies Institute on Transmission Electron Microscopy in Materials Science July 21, 2006 ACKNOWLEDGMENTS • • • • • Nihon University, Tokyo, Japan A. Fukami A. Ishikawa H. Konishi H. Miyata • • • • • Northwestern University, Evanston Y.-C. Lee R. C. Mucic C. A. Mirkin D. F. Shriver JEOL, LTD., Tokyo, Japan K. Fukushima University of California, Irvine K. Foo L. Lai Naval Research Laboratory, USA R. H. Bennett OUTLINE INTRODUCTION Electron Microscopy The Environment Purposes Historical Review of EC Research Types of In-Situ Observation/Research How to Maintain Original Hydrated State in the EM Methods of Containing the Liquid Environment EC TEM MATERIALS and TEM RESULTS Inert Fillers Clay Minerals DNA/Au Nanoparticles SUMMARY INTRODUCTION Electron Microscopy - Daily tool in our modern day research and development - Routine for many laboratory (e.g., hospital, semi-conductor industry - Used in all scientific and engineering field research Particle Size The range in size of grain and/or particles is very great. At the lower end of the scale: may be a component part of an atom The upper end of the scale: is not fixed. Scientists always ask what is the grain/particle size? Effect of size on various properties of particles/materials The most outstanding property : surface-to-mass ratio. stress σ The utilization of inorganic nano-particles demonstrated improvements of a large number of physical and mechanical properties (modulus, strength, thermal expansion coefficient), barrier, flammability resistance etc. modified Unmodified Conventional Material Nanocomposits Strain ε What is the Real world? - Are they real in the EM? - Vacuum Environment - Sample Preparation - Artifacts - Result and Interpretation - Understanding of instrumentation & science The Environment • Earth, an unique place in the solar system. - is probably the only planet has water and life, though scientists have been searching for other possible life in the universe. • All kinds of LIFE do need H2O, Our daily life needs water: food, farming, cement, ……. • However, a few things do not like water. For examples: Electron – Does Not Like H2O, so as electron Microscope • So we all have to dehydrate our samples before we insert our samples in to the EM. • Dehydration – Undesirable Structural and Morphological modification How to See the Real World? In Situ – Examining materials at the original state/environment. – the first step for Dynamic Experiments (in an electron microscope) – provide a unique and powerful method of studying materials, especially the response/reaction of materials to the change of the environment to understand the fundamental mechanism of any chemical interaction. e. g., Chemical reaction at elevated temperatures Gas – solid interaction Liquid – solid interaction Liquid – liquid interaction Type of In Situ Observation/Research 1. In Situ Deformation 2. In Situ High Temperature Microscopy 3. In Situ Low Temperature Microscopy 4. In Situ Studies of Gas – Solid Interaction 5. In Situ Environmental (Wet) Cell Microscopy 6. In Situ Studies of Vapor Deposition Purposes 1. To introduce the (wet) environmental (cell, EC) transmission electron microscopy (TEM) 2. To show some research results obtained from EC TEM 3. To share my thoughts with you and to listen your ideas and comments Historical Review of Environmental Cell Research Since 1935 Marton tried to examine biological materials in hydrated state…. 1944 Abrams and McBain constructed the first enclosed wet cell Only a very few researchers carried out EC research in the past. The main reasons: 1. Difficulties on instrumentation. 2. Difficulties on resolution 3. Funding Between mid-60 through late 70 probably have the most activities. • However, it has attracted much attention in the EC TEM research in the last 3-5 years though they were only limited to EGC TEM research. Europe: Stoyanova, I. G., in Russia (late 1950 to early 1960) on biological sciences (gas and wet cell), first to examine wet biological materials in TEM Heide, H. G., in Germany (early 1960) on Siemens Elmiskope, biological science (gas controlled environment) Escaig, J., and Sella, C., in France (mid-1960 to early 1970) on physical sciences (gas-solid) Dupoy, G., in France (early – late 1960) on physical sciences (wet cell) Swan, P., and Tighe, N. J., in England (during 1970) on physical sciences (more on gas-solid interaction) Flower, H. M., (mid 1970) on materials science (gas-solid interaction, corrosion) Gai, P. L., (late 1970 to present) in England and in US (after mid1980) on materials science (gas-solid interaction, chemical reaction, catalysts). USA: Allison, D. L. (early to mid 1970) on physical sciences Hui, S. W., (during 1970 to early 1980) on biological sciences Moretz, R. C. (late 1960 to early 1970) on biological sciences , Parsons, D. F., (early to late 1970) on physical aspect of biological samples. Gai and Boyes, Du Pont Research (after 1980) on materials aspect, most work on catalyst research (gas interaction). ASU group (after early 1990) on the materials science aspect, recent work concentrated on catalyst research (gas interaction). Japan: Ito, T (late 1950) on physical sciences/Instrumentation (gas interaction) Hashimoto, H., (late 1950 through early 1970), on physical sciences (gas interaction) Fukami, A., (late 1960 through late 1980) on physical and biological sciences and instrumentation (liquid wet cell, liquid-liquid interaction) Three individual groups, Doi, M, Fujita, H., Nagata, F., and Sakata, S., (early to mid-1970) on instrumentation and HVTEM How to maintain the ORIGINAL (HYDRATED) STATE in the EM? Principle: The phase-temperature phase diagram for water indicates that true “wet” conditions only exist at pressures of at least 600 Pa at 0 oC (4.6 Torr = 4.6 mm Hg). In the range 650 to 1300 Pa (5 –10 Torr) the specimen may be observed while at equilibrium with water. SEM – Environmental SEM (ESEM),Variable Pressure SEM (1-270 Pa) – Natural SEM, Partially hydrated – SEM operates at 50 – 90 Pa TEM- 1. Gas 2. Wet Cell (Liquid/Aqueous Environment) 3. Both gas and liquid Well Cell – A specimen chamber which is capable of controlling the environment surround the specimen, but the main microscope vacuum remains undisturbed. Methods of Containing the Liquid Environment 1. Window Technique A pair of electron transparent windows can be placed above and below the specimen. The specimen and its surrounding gas/liquid is completely sealed off from the EM column so the pressure/vacuum of the EM remains constant. Need sufficient strong (often thicker) window/film to resist the pressure difference between the cell and EM, but the resolution and contrast of image were not seriously degraded. Risk: Damage of window, possibility of contamination to the microscope or even chemical attack on the microscope. The only choice for liquid environment and liquid chemical interaction research. This technique can achieve maximum pressure in the cell. 2. Aperture-Limited Technique A pair of single or multiple small-bore aperture can be placed above and below the specimen. Use differential pumping technology. Gas leakage from around the specimen into the column occurs. Microscope vacuum is controlled by the size of apertures for differential pumping A dynamic balance (equilibrium) between: 1. the gas flow into the cell; 2. the leak rate through the cell aperture; 3. differential pumping aperture; and 4. the pumping speed of the microscope Transmission Electron Microscopy (used in this research) (I) Modified TEM (JEOL 2000FX) (II) Specially Constructed Wet Environmental Cell (EC) (TEM sample holder) (III) TEM/EC Vacuum Controller EC -TEM JEOL JEM-2000 EX TEM with side-entry EC holder and gas/liquid control system A large side-entry specimen holder for EC observation: (A) cover, (B) inner pipes, and (C) sealing block Detail Diagram of EC Sample Stage EC TEM Environmental Control System Diagram of gas-liquid environmental control system for in-situ wet cell TEM RESULTS (1) (I) Polymer Electrolytes • Potential application in electrochemical devices. • Composed of conventional polymer electrolyte and inert fillers, provide an avenue to enhance mechanical strength while maintain ionic conductivity, if the particle sizes of these inert fillers are sufficient small (< 1um in diameter). • To determine the morphology/size of inert fillers to further our understanding of ion transport phenomena in the composite system. Material: Surface modified fume silica (7 um) (a) SiCl4 was used to activate surface silanol (SiOH) group and generate reactive groups on the surface of fume silica particles (b) Sodium isethionate (HOC2H4SO3Na) interacted with SiCl groups on the surface to produce surface-modified fume silica particles. Na = 0.4 Wt.% (c) (i) Dispersed in tetraethylene glycol dimethyl ether tetraglyme, H3C(OC2H4)4OCH3) (ii) Dispersed in water Original fume silica particles (CTEM) Untreated fume silica particles (HREM) Morphology of surface-modified silica particles (CTEM) Surface-modified silica particles (HREM) Fume silica particles in tetraglyme medium (In-situ EC TEM) Aggregated silica particles after removal of tetraglyme medium (In-situ EC-TEM) Silica particles in water (In-situ EC-TEM) Silica particles after evaporation of water (In-situ EC-TEM) Discussion/Summary: • The difference in morphologies in two medias may originated from the difference of polarity and the sodium content. • Apparently, the sodium cations on the surface produce a moderate increase in the hydrophilicity of the particles, therefore, high dispersion was observed in tetraglyme medium. • The relatively low loading of Na cations on the silica surface prevented full dispersion of silica particles. RESULTS (2) (II) Clay (Smectite/Montomorillonite) Particles - Ubiquitous on the Earth. Important in our daily life: geology, petroleum industry; soil and crop sciences, Materials science/engineering Filler, polymer/nanoclay composites…………….. - Layered Al silicate, traditionally believed as broad undulating mosaic sheets, irregular fluffy masses of extremely small particles, irregular flake-shaped or platy platelets. - Objective: To study the particle size/shape analysis in H2O. - Method: a) Purified (ion exchanged with Na) and size sieved through certain size (< submicron) b) Dispersed in de-ionized water Question: Are They Real in EM? • Problems/Difficulties (of examining smetitic clays): (a) The individual particles can barely be discerned and are too small to reveal any characteristic outlines. (b) Estimations of the areal dimensions are difficult because of the irregularity (c) Clays love H2O - Disperse in H2O - Aggregate when they dry 500 nm 500 nm Aggregated smectite clay particles (CTEM) Smecitite clay particles in de-ionized water (In-Situ EC-TEM) Well-dispersed smectite particles in de-ionized water (EC TEM) Close-up view of dispersed clay particles (EC TEM) Particle size analysis 30 25 Individual % 20 15 10 5 0 500 400 300 200 100 100 80 60 40 20 50 40 30 20 10 Particle size (nm) 0 Diameter (nm) 10 2 AR1 (Tc = 5nm) 5 1 Ar2 (Tc = 10nm) Aggregates formed after evaporation of water in EC TEM EC TEM micrograph depicting small needle-like and rounded particles rest on the surface of large platelets. ED of smecitie clay particle (In-Situ TEM) High magnification EC TEM revealing elongated thin clay laths Low magnification EC TEM image showing mixed texture of smectite particles. EC TEM image revealing not completely dispersed clay particles. Note the spherical/ball-shape aggregates with empty space inside the aggregates. Discussion/Summary: 1. Based on EC TEM study it is clear that the morphology of smectitic clay minerals is not only existed as plate-like morphology which has traditionally been proposed (and observed in a conventional TEM). 2. Instead there are different shapes and sizes. A variety of shape such as platelets, needle-like, thin lath, disc and/or spherical, polygon shape, have been observed in EC TEM. 3. With well-dispersed clay particles, particle size analysis can be carried out much easier (without any ambiguity in differentiating particle shape and boundary) RESULTS (3) (III) DNA/Au Nanoparticles -The assembly of nanometer sized building blocks, DNA/nanoparticle hybrid materials and assemblies might have useful electrical, optical and structural properties. Objective: to observe in situ observation of DNA/Au nanoparticle assembles in liquid media Material: Citrate-stabilized Au particles, 8 and 31 nm in diameter Method: a) 8 nm Au particles were modified with propylthiol-capped oligonucleotide, 3’HS(CH2)3-O(O-)P(O))-ATG-CTC-AACTCT b) 31 nm particles were modified with hexlylthiol-capped oligonucleotide, 3”TAG-GAC-TTA-CGC-O(O)P(O)O(CH2)6SH c) The assembly strategy for the oligonucleotide-modified particles was based on the ability of oligonucleotide to link the particle together. Binary particles network assembly (CTEM) DNA/Au nano-particles satellite structure (CTEM) Dispersed binary particles without linking olignucleotide (CTEM) EC TEM image showing DNA/Au assembly in more 3-dimensional structure Dissociation of DNA/Au nano-particles linkages (2) (In-Situ EC-TEM) Breakdown of DNA/Au particles linkages (1) (In-Situ EC-TEM) Dispersed Au particles after electron irradiation (In-Situ EC-TEM) Dynamic movement of Au nano-particles (In-Situ EC-TEM) indicating the existence of liquid in the EC. In-Situ TEM image revealing grain aggregation after prolonged electron irradiation (continuously increasing the temperature in EC) without oligonucleotide. Discussion/Summary: -Melting analysis of the nanoparticle-modified surface showed that oligonucleotide hybrization, which is responsible for the linkage of Au nanoparticles, can be destroyed when temp.> 52 oC. *Dispersion of Au nanoparticles without any specific linkage or strands: -Unstable hybridization and dissociation of DNA/AU linkage due to the increased temperature in EC by electron beam heating; - Damage of DNA after prolonged electron irradiation *Grain growth and aggregation without oligonucletide: -Continuously increasing the temperature in the EC Salt precipitates after removal of water (In-Situ TEM) SUMMARY 1. These experiments have demonstrated the importance of environmental (EC) TEM that not only allows dynamic observation but also provides important research information. 2. More researches on EC instrumentation are need: (a) Stage – sample loading (b) Vacuum control on EC – computerize control (c) Resolution (d) Microchemical analysis 3. With the EC TEM, more research and new discovery is awaiting us to explore. The EC TEM is a gold mine to many scientific (physical, biological, and medical) and engineering researches. 謝謝 Thank You Muchas Gracias References (1) (2) (3) (4) Information and micrographs presented herewith were taken from the research results of the following papers: W.-A. Chiou et. al., In Situ TEM Study of Inert Fillers in Liquid Environment: Microsc. Microanalysis (Suppl. 2), MSA, 1998. W.-A. Chiou et. al., In Situ TEM Study of DNA/Gold Nanoparticles in Liquid environment: Microsc. Microanalysis (Suppl. 2), MSA, 1999. W.-A. Chiou et al., Fundamental Thickness of Smectitic Clay Particles, 13th International Clay Conference, Tokyo, Japan, August, 2005. References cited on the above papers.