Carbohydrates
advertisement

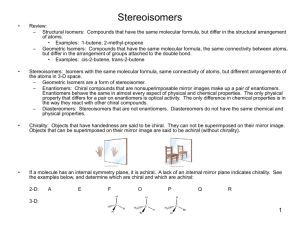
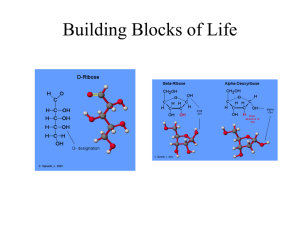
Carbohydrate Basics Carbohydrates (Overview): - essential components of all living things. - most abundant of the four major classes of biological molecules (other classes are proteins, nucleic acids and lipids). - range in complexity from simple 3-carbon sugars to enormous complexes containing hundreds of polysaccharide chains linked to a protein core. - serve as metabolic intermediates, energy stores, and fuels. - components of DNA and RNA (ribose and deoxyribose). - major structural components of plants and animals - bound to proteins (glycoproteins) and lipids (glycolipids). - are important for an enormous number of vital cellular functions, including cell-cell recognition and signaling. Aldoses & Ketoses Chirality & Optical Activity The term chiral is used to describe an object (molecule) which is non-superimposable on its mirror image. Optical activity is the ability of a chiral molecule to rotate the plane of plane-polarized light, measured using a polarimeter. A simple polarimeter consists of a light source, polarizing lens, sample tube and analysing lens. * * * * Emil Fischer Enantiomers and Diastereomers Molecules with the same molecular formulas and order of attachment of constituent atoms but which differ in the arrangement of these atoms in space are called stereoisomers. Enantiomers are stereoisomers that are mirror images of each other. The mirror image of an enantiomer cannot be superimposed on itself. In general, enantiomers have identical chemical and physical properties and racemic mixtures of enantiomers are difficult to separate into the individual enantiomers. Diastereomers are stereoisomers that are not mirror images of each other. In general they have different chemical and physical properties and can be separated from each other. If they differ at one chiral center then they are epimers. O H C HO C H H C OH O H C H C OH HO C H HO C H HO C H CH2OH H H L-Gluc ose C OH C OH CH2OH Enantiomers D-Glucose Mi rr or p la n e O C H H C OH H C OH HO C H HO C H CH2OH L-Man nose O H C H C OH HO C H H C OH H C OH Diastereomer s CH2OH D-Glucose Racemic mixtures of enantiomers are often separated by derivatizing the mixture with a chiral reagent thereby converting the components to a mixture of diastereomers which can then be separated. The original compounds are then regenerated. Resolution of a Racemic Mixture Jean Baptiste Biot's young protegé, Louis Pasteur, was the first person to separate a racemic mixture of optical isomers. He initially used tweezers to physically separate left- and right-handed crystals of tartaric acid. Pasteur subsequently devised the still widely used procedure for resolving a mixture of optical isomers based on converting them to diastereomers with different chemical and physical properties. After separation of the diastereomers the original optical isomers are then regenerated.. Louis Pasteur Oxidation: CHO ~~~ Br2 H2O COOH an aldonic acid ~~~ Ketoses, such as fructose, are not oxidized. However, in alkaline solution both aldoses and ketoses are oxidized by either Ag+ (to give metalic silver) or by Cu+2 to to give Cu+. “reducing sugars” Another oxidation: CHO COOH dil. HNO3 CH2OH COOH An aldaric acid Reduction: CHO NaBH4 ~~~ ~~~ CH2OH CO ~~~ CH2OH an alditol CH2OH NaBH4 OH ~~~ CH2OH + HO ~~~ Fig. 7-05, p.207 Fig. 7-05, p.207 Fehlings reaction for aldehyde (can also detect ketones after tautamerization) Fischer Projection Formulas Fig. 7-06, p.208 Fig. 7-06, p.208 Fig. 7-06, p.208 Fig. 7-06, p.208 An omeric Co n figu ratio n s (Co n t'd) Th e an o m er h av in g t h e sam e co n figu r at io n , in t h e Fisc h er p r o ject io n , at t h e an o m er ic an d r efer en ce c ar b o n (h igh est n u m b er ed asy m m et r ic car b o n ) is d esign at ed In t h e case o f ald o h exo p y r an o ses, t h e C-6 su b st it u en t is o n t h e sam e sid e o f t h e r in g as t h e an o m er ic h y d r o xy l gr o u p in t h e an o m er an d o n o p p o sit e sid e s in t h e an o m er . CH2OH O O OH CH3 OH HO - D-Glucopyranose CH3 OH HO OH O OH HO OH -L-Fucopyr anose OH OH OH -L -Fucopyr anose Possible conformations: 2 chairs 4 boats 6 skews 12 half chairs * p.213 Common Alditols Amino Sugars • Sugars with an amino group at C-2 are amino sugars. • They are found in many oligosaccharides and polysaccharides. Figure 7.14 Muramic acid Muramic acid is a component of the polysaccharides of cell membranes of higher organisms and also bacterial cell walls. Muramic acid is a glycosamine linked to a 3-carbon acid at C-3. (Murus is Latin for “wall”.) Figure 7.15 Structure of muramic acid. Sialic acids The N-acetyl and N-glycolyl derivatives of neuraminic acid are known as sialic acids. Figure 7.15 Two depictions of a sialic acid. Fig. 7-19, p.218