Chapter 3 Quiz - Parkway C-2
advertisement

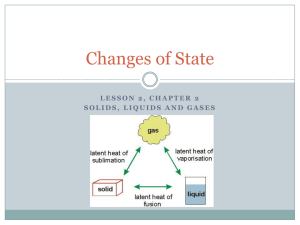
Chapter 3 Quiz By: Mohammed Khezri & Sophia Baik Definition #1 • The result of a force distributed over an area is…… Definition #1 • The result of a force distributed over an area is Pressure. Definition #2 • The direct proportion of the volume of a gas to temperature (in kelvins) if the pressure and the number of particles are constant it is ……. Definition #2 • The direct proportion of the volume of a gas to temperature (in kelvins) if the pressure and the number of particles are constant is Charles’s Law. Definition #3 • The inverse variation of the volume of gas with its pressure if the temperature and the number of the particles are constant is... Definition #3 • The inverse variation of the volume of gas with its pressure if the temperature and the number of the particles are constant is Boyle’s Law. Definition #4 • A description of a change in which a system absorbs energy from its surroundings is ….. Definition #4 • A description of a change in which a system absorbs energy from its surroundings is called endothermic. Definition #5 • The energy a substance must absorb in order to change from a solid to liquid is called….. Definition #5 • The energy a substance must absorb in order to change from a solid to liquid is called Heat of Fusion. Definition #6 • A description of a change in which a system releases energy to its surroundings is …. Definition #6 • A description of a change in which a system releases energy to its surroundings is Exothermic. Definition #7 • The energy a substance must absorb in order to change from a liquid to a gas is… Definition #7 • The energy a substance must absorb in order to change from a liquid to a gas is heat of vaporization. Definition #8 • The state of matter in which a material has neither a definite shape or volume is….. Definition #8 • The state of matter in which a material has neither a definite shape or volume is gas. Definition #9 • The phase change in which a substance changes directly from a solid to a gas or vapor without changing to a liquid first is…. Definition #9 • The phase change in which a substance changes directly from a solid to a gas or vapor without changing to a liquid first is vaporization. Definition #10 • The phase change in which a substance changes from a solid to a gas or vapor without changing to a liquid first is…. Definition #10 • The phase change in which a substance changes from a solid to a gas or vapor without changing to a liquid first is sublimation. What is this an example of? This is an example of a SOLID. What is this coming out of the tap? Its Liquid coming out of the tap!!! What type of energy does this object have? Kinetic Energy Kinetic Energy is energy an object has when its in motion. What is a type of matter which is not a solid liquid or a gas? PLASMA Plasma is a state if matter in which atom have been stripped of their electrons. It’s so hot on the sun that the liquid looking material is plasma. What is this Physical Change called? Phase Change Phase change is a physical change that occurs when one substance changes from a state of matter to another state of matter. What is happening to the liquid? EVAPORATION. Evaporation is the process that changes a substance from a liquid to gas at the temperature of the liquids boiling point. What happens after the water in the left container starts evaporating? In the second flask the vapor pressure starts to form. Vapor Pressure is the Pressure that is caused by the collisions of particles in a vapor with the walls of the container. What is the phase called when it starts to rain? Condensation is when any gas turns into a liquid. The best example is when it rains. The clouds (which are gas) precipitate and the gas turns into liquid This is an example of..? DEPOSITION. • Deposition causes frost to form on windows; When the water vapor in the air comes in contact with cold window glass, the water vapor loses enough kinetic energy to change directly from a gas to a solid. A temperature of 0 Kelvins is also called..? ABSOLUTE ZERO. The six common phase changes are: • A) melting, freezing, vaporization, evaporation, condensation, deposition • B) melting, freezing, vaporization, condensation, sublimation, deposition • C) melting, freezing, boiling, vaporization, condensation, sublimation • D) melting, freezing, condensation, sublimation, deposition, conductivity The six common phase changes are: • A) melting, freezing, vaporization, evaporation, condensation, deposition • B) melting, freezing, vaporization, condensation, sublimation, deposition • C) melting, freezing, boiling, vaporization, condensation, sublimation • D) melting, freezing, condensation, sublimation, deposition, conductivity What are the factors that affect the pressure of an enclosed gas? • A) its temperature, its mass, and size of its particles • B) its mass, its volume, and size of its particles • C) its temperature, its volume, and number of its particles • D) its mass, its volume, and number of particles What are the factors that affect the pressure of an enclosed gas? • A) its temperature, its mass, and size of its particles • B) its mass, its volume, and size of its particles • C) its temperature, its volume, and number of its particles • D) its mass, its volume, and number of particles Charles’s Law can be written as a mathematical expression of; • A) V1/T1=V2/T2 • B) P1V1=P2V2 • C) V1/V2=T1/T2 • D) P1V2=P2V1 Charles’s Law can be written as a mathematical expression of; • A) V1/T1=V2/T2 • B) P1V1=P2V2 • C) V1/V2=T1/T2 • D) P1V2=P2V1 At extremely high temperatures, matter exists as: • A) Gas • B) Vapor • C) Bose-Einstein Condensate • D) Plasma At extremely high temperatures, matter exists as: • A) Gas • B) Vapor • C) Bose-Einstein Condensate • D) Plasma Solids have a definite volume and shape because: • A) the particles fixed locations • B) the particles • C) the particles close together • D) the particles random vibrate around don’t move at all are packed are arranged in Solids have a definite volume and shape because: • A) the particles fixed locations • B) the particles • C) the particles close together • D) the particles random vibrate around don’t move at all are packed are arranged in What causes the pressure in a closed container of gas? • A) The fast, vibrating motion of the particles of the gas • B) The collisions between particles of the gas and the walls of the container • C) The force with which the particles of the gas hit the walls of the container • D) The number of particles in the gas What causes the pressure in a closed container of gas? • A) The fast, vibrating motion of the particles of the gas • B) The collisions between particles of the gas and the walls of the container • C) The force with which the particles of the gas hit the walls of the container • D) The number of particles in the gas During the Phase Change what does NOT change in an object? • A) Temperature • B) Chemical Properties • C) Physical Properties • D) Corners During the Phase Change what does NOT change in an object? • A) Temperature • B) Chemical Properties • C) Physical Properties • D) Corners What becomes more orderly as water freezes? • A) Arrangement of Molecules • B) Arrangement of Oxygen • C) Arrangement of hydrogen • D) none of the above What becomes more orderly as water freezes? • A) Arrangement of Molecules • B) Arrangement of Oxygen • C) Arrangement of hydrogen • D) none of the above Reducing the volume of a gas increases its pressure if the temperature of the gas and the number of particles are • A) Constant • B) Moving • C) Frozen • D) All of the above Reducing the volume of a gas increases its pressure if the temperature of the gas and the number of particles are: • A) Constant • B) Moving • C) Frozen • D) All of the above What states all particles of matter are in constant motion? • A) Charles’s Law • B) Boyle’s Law • C) Kinetic Theory • D) Newton’s Law What states all particles of matter are in constant motion? • A) Charles’s Law • B) Boyle’s Law • C) Kinetic Theory • D) Newton’s Law THE END A