Answer Key to week 11/03
advertisement

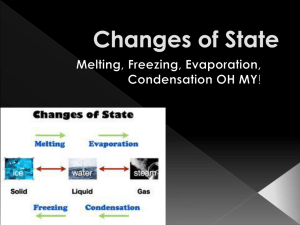
NAME Answer Key Date__________________ HOMEROOM_____________________ SCIENCE Practice QUIZ Chapter 2 Relax! Take a deep breath! Count backwards from 10! Now take your quiz! 1. Which process occurs when a liquid changes to a gas? a) condensation b) evaporation c) freezing d) melting 2. Sublimation occurs when a solid changes directly to a gas. (condensation, sublimation.) True or False: 3. False When a chemical change occurs, matter keeps the same chemical properties. 4. True When matter changes state, it is a physical change. 5. True Forces between particles in a solid keep them from moving to a new place. 6. False Gases always have the same shape and volume. 7. Compare the motion and spacing of particles as materials cools form a gas to a liquid and then to a solid phase. Do particles ever stop moving? As materials cool from a gas to a liquid and then to a solid phase, particles will slow down and come close to each other. Particles never stop moving unless they get to the Bose Einstein Condensate or absolute Zero. 8. Give two examples of a physical change: Answers will vary Shredding paper, breaking a pencil, crushing graham crackers, making origami, etc. 9. Draw a picture of what particles look like in a solid, in a liquid, and a gas. 10. You take a cold can of juice from the refrigerator on a hot, humid day. Predict whether condensation will occur on the outside of the can. Explain what will happen to the particles. Condensation will take place on the outside of the can because the water vapor in the air will touch the cold surface of the can and give up some heat energy. The water molecules will slow down and come together on the surface of the can. Liquid drops will form as more water molecules come together