Folie 1 - Clinical Trial Results
advertisement

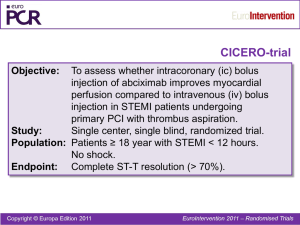
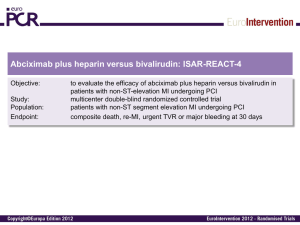
ISAR-REACT 2 One-year Clinical Outcomes in the ISARREACT 2 Trial, a Randomized Comparison of Abciximab Versus Placebo in Patients With non-ST Segment Elevation Acute Coronary Syndromes Undergoing PCI After Pretreatment With Clopidogrel M. Seyfarth, A. Kastrati, J. Mehilli, F.-J. Neumann, J. ten Berg, O. Bruskina, F. Dotzer, J. Pache, J. Dirschinger, P. B. Berger, A. Schömig ESC 2007 ISAR-REACT 2 Presenter Disclosure Information ISAR-REACT 2 Trial Study performed without industry support The following potential of conflict exist related to this presentation: Seyfarth - Lecture fees from BMS, Lilly, Sanofi-Aventis: Modest level relationship Kastrati - Lecture fees from BMS, Lilly, Sanofi-Aventis: Modest level relationship Mehilli - No relationships to disclose Neumann - No relationships to disclose ten Berg - No relationships to disclose Bruskina - No relationships to disclose Dotzer - No relationships to disclose Pache - No relationships to disclose Dirschinger - No relationships to disclose Berger - Lecture Fees from Schering Plough and from CME: Modest level relationship Schömig - Unrestricted Grant from BMS and Nycomed: Modest level relationship ESC 2007 ISAR-REACT 2 ISAR-REACT 2 Multicenter, randomized, double-blind, placebo-controlled trial 2022 patients with NSTE-ACS Clopidogrel 600 mg at least 2h before procedure; Aspirin i.v. Abciximab Heparin 70 U/Kg Abciximab (bolus & 12h infus.) Placebo Heparin bolus of 140 U/Kg Placebo (bolus & 12h infus.) Clopidogrel 2x75 mg/day until discharge 75 mg for at least 4 weeks Aspirin 200 mg/day ISAR-REACT 2, JAMA 2006 ESC 2007 ISAR-REACT 2 ISAR-REACT 2: Inclusion Criteria • Rest anginal episodes in the last 48 hours with • An elevated troponin level (>.03 mg/L) or • ST-segment depression ISAR-REACT 2, JAMA 2006 ESC 2007 ISAR-REACT 2 ISAR-REACT 2: Exclusion Criteria • ST-elevation acute MI • Hemodynamic instability • Pericarditis • Increased risk of bleeding, malignancies • Relevant hematologic deviations • Known allergic reaction to the study medication • Pregnancy (present or suspected) ISAR-REACT 2, JAMA 2006 ESC 2007 ISAR-REACT 2 ISAR-RACT 2: Primary End Point A composite of death, MI or urgent target vessel revascularization within the first 30 days after PCI. ISAR-REACT 2, JAMA 2006 ESC 2007 ISAR-REACT 2 Primary Endpoint of ISAR-REACT-2 Death/MI/TVR 15 Placebo 11.9 Abciximab 8.9 % 10 5 P=.03 RR 0.75 [0.58-0.97] 0 0 5 10 15 20 25 30 Days after randomization ISAR-REACT 2, JAMA 2006 ESC 2007 ISAR-REACT 2 Objective of the present study to investigate whether benefits of abciximab are maintained at 1 year after PCI in patients with NSTE-ACS enrolled in the ISAR-REACT 2 trial. ESC 2007 ISAR-REACT 2 Follow-Up Protocol 600 mg Clopidogrel PCI Abciximab vs. Placebo 0 serial CK + CKMB measurements 30 d 6 mo clinical follow-up clinical follow-up 12 mo. clinical follow-up ESC 2007 ISAR-REACT 2 Baseline Clinical Characteristics Abciximab n=1012 Placebo n=1010 66.0±11.0 66.5±11.3 Women, % Hypercholesterolemia, % 23.3 61.6 25.9 60.3 Arterial hypertension, % Diabetes mellitus, % 62.5 24.9 64.3 28.1 Current smoker, % Body mass index, kg/m2 22.7 21.7 Age, yrs 27.2 ±3.9 27.3±4.2 ESC 2007 ISAR-REACT 2 Baseline Clinical Characteristics (con‘t) Abciximab n=1012 Placebo n=1010 53.3±12.3 53.3±12.5 Multivessel disease, % Prior MI, % 74.4 24.2 74.3 24.1 Prior CABG, % Elevated troponin, % 10.1 50.7 10.8 53.1 Ejection fraction, % ESC 2007 ISAR-REACT 2 Lesion Characteristics Abciximab n=1012 Vessel LCA, % LAD, % LCx, % RCA, % Bypass graft, % Complex (B2/C) lesions, % DES, % BMS, % PTCA, % 2.4 41.9 23.8 28.1 3.8 80.2 49.5 47.8 2.7 Placebo n=1010 2.2 40.4 26.0 26.2 5.2 81.2 48.9 47.6 3.5 ESC 2007 ISAR-REACT 2 Primary Endpoint after 12 Months - Survival free of MI and TVR 100 % 90 Abciximab 80 70 P=0.012 60 Placebo RR 0.80 [0.67-0.95] 50 0 1 2 3 4 5 6 7 8 9 Months after randomization 10 11 12 ESC 2007 ISAR-REACT 2 Primary Endpoint after 12 Months - Survival free of MI 100 % Abciximab 90 80 Placebo 70 P=0.015 60 RR 0.74 [0.59-0.94] 50 0 1 2 3 4 5 6 7 8 9 Months after randomization 10 11 12 ESC 2007 ISAR-REACT 2 Subset Analyses Abciximab No./Total (%) All Patients Relative Risk Placebo No./Total (%) 234/1012 (23.3) 281/1010 (28.0) 128/482 (26.6) 106/530 (20.2) 159/527 (30.3) 122/483 (25.5) 51/236 (21.7) 183/776 (23.8) 71/262 (27.4) 210/748 (28.2) 68/252 (27.1) 166/760 (22.0) 80/284 (28.6) 201/726 (27.8) 146/513 (28.6) 88/499 (17.8) 178/536 (33.3) 103/474 (22.0) 93/475 (19.8) 141/537 (26.4) 115/461 (25.1) 166/549 (30.4) Age >67 years ≤67 years Sex Women Men Diabetes Yes No Troponin >0.03 mg/L Yes No Clopidogrel interval >3 hours ≤3 hours 0.5 0.6 0.7 0.8 0.9 1.0 1.1 1.2 1.3 ESC 2007 ISAR-REACT 2 Troponin Level and Benefit With Abciximab Death/MI/UTVR, % 20 Troponin-Positive: RR=0.71 [0.54-0.95] 15 10 Abciximab vs. Placebo Troponin-Negative: RR=0.99 [0.56-1.76] 5 0 0 5 10 15 20 25 30 Days after randomization ESC 2007 ISAR-REACT 2 Troponin Level and Benefit With Abciximab after 12 Months 100 % 90 Troponin level ≤0.03 µg/L 80 P=0.10 70 P=0.07 Troponin level >0.03 µg/L Abciximab 60 Placebo 50 0 1 2 3 4 5 6 7 8 9 Months after randomization 10 11 12 ESC 2007 ISAR-REACT 2 Efficacy Analysis According to Troponin Level Abciximab Placebo 18 17.1 16.8 % 12.7 15.5 13.8 13.2 12 6.6 6.7 4.6 5.1 6 2.2 2.7 0 Death MI TVR Troponin level >0.03 µg/L Death MI TVR Troponin level ≤0.03 µg/L ESC 2007 ISAR-REACT 2 Conclusions The early benefit of Abciximab in patients with NSTEACS undergoing PCI after pretreatment with 600 mg Clopidogrel is maintained at 1 year after administration. Another novel finding of this 1-year analysis is the additional benefit of Abciximab in low-risk patients without an elevated troponin in terms of a reduction of target vessel revascularization. ESC 2007