Chapter 3 Presentation
advertisement

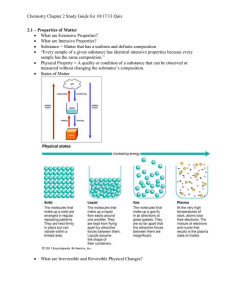
Chapter 3 Matter – Properties and Changes Section 1 Properties of Matter Section 1: Properties of Matter Most common substances exist as solids, liquids, and gases, which have diverse physical and chemical properties. K What I Know W What I Want to Find Out L What I Learned Substances Matter is anything that has mass and takes up space. Matter is everything around us. Matter with a uniform and unchanging composition is a substance. Much of your chemistry course will be focused on the composition of substances and how they interact with one another. Properties of Matter States of Matter The physical forms of matter, either solid, liquid, or gas, are called the states of matter. Solids are a form of matter that have their own definite shape and volume. Liquids are a form of matter that have a definite volume but take the shape of the container. Properties of Matter States of Matter Gases have no definite shape or volume. They expand to fill their container. Vapor refers to the gaseous state of a substance that is a solid or liquid at room temperature. Properties of Matter Physical Properties • Let’s play 20 Questions! QUESTIONS QUESTIONS 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Physical Properties of Matter A physical property is a characteristic that can be observed or measured without changing the sample’s composition. -ex: viscosity, hardness, density, malleability, melting point, boiling point, etc. Properties of Matter Physical Properties of Matter Extensive properties, such as mass, length, and volume, are dependent on the amount of substance present. Intensive properties, such as density, are dependent on the what the substance is not how much there is. Properties of Matter Chemical Properties of Matter The ability of a substance to combine with or change into one or more other substances is called a chemical property. Examples include: • Iron forming rust • Copper turning green in the air • Flammability • Reactivity Properties of Matter Observing Properties of Matter A substance can change form—an important concept in chemistry. Both physical and chemical properties can change with specific environmental conditions, such as temperature and pressure. Properties of Matter Physical & Chemical Properties Physical & Chemical Properties Song Essential Questions • What characteristics identify a substance? • What distinguishes physical properties from chemical properties? • How do the properties of the physical states of matter differ? Properties of Matter Section 2 Changes in Matter Section 2: Changes in Matter Matter can undergo physical and chemical changes. K What I Know W What I Want to Find Out L What I Learned Physical Changes A change that alters a substance without changing its composition is known as a physical change. A phase change is a transition of matter from one state to another. Boiling, freezing, melting, and condensing all describe phase changes in chemistry. Changes in Matter Phase Changes • Phase change- reversible physical change that occurs when a substance changes from one state of matter to another. • Melting, freezing, vaporization, condensation, sublimation, and deposition are the six common phase changes. Energy and Phase Changes • During a phase change, energy is transferred between a substance and its surroundings. • Energy is either absorbed or released during a phase change. MELTING • Heat flows from the air to the ice. As ice gains energy, the molecules vibrate more quickly. At the melting point of water, 0ºC, some molecules have enough energy to overcome the attractions and move. • When all the molecules have enough energy to move, melting is complete. Any additional energy will cause the temperature to rise. FREEZING • When liquid is placed in a freezer, energy flows from the liquid to the air in the freezer. The water cools down. Kinetic energy decreases, particles move more slowly and begin to arrange themselves in an orderly pattern. • Freezing is exothermic because it gives off energy to the surroundings. • Freezing does not mean “cold.” Silicon freezes at 1412 ºC VAPORIZATION • When a substance changes from a liquid to a gas. The substance must absorb energy. In a refrigerator, a pair of phase changes keep the food cold. Energy from inside the food compartment is used to change a liquid to a gas in the evaporator. This energy is released when the compressed gas changes back to a liquid in the condenser Condensation • CONDENSATION – The phase change in which a substance changes from gas to liquid. Water vapor from the air condensed into drops of liquid water on these blades of grass More Phase Changes • SUBLIMATION – Phase change in which a substance changes from a solid to a gas without first changing to a liquid. Ex: Dry ice • DEPOSITION – Phase change when a gas changes directly into a solid without first changing to a liquid. Ex: Frost The trap is baited with dry ice because mosquitoes are attracted to carbon dioxide. Chemical Changes A change that involves one or more substances turning into new substances is called a chemical change. Decomposing, rusting, exploding, burning, or oxidizing are all terms that describe chemical changes. Changes in Matter Physical vs Chemical Change http://www.youtube.com/watch?v=g Cbqjs-pqJo Gummy Bear torture http://www.youtube.com/watch?v=u j9D3mc7tVg Law of Conservation of Mass The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction, it is conserved. The mass of the reactants equals the mass of the products. massreactants = massproducts Changes in Matter CONSERVATION OF MASS KNOWN UNKNOWN mmercury(II) oxide = 10.00 g moxygen = ? g EXAMPLE mmercury = 9.26 g Problem In an experiment, 10.00 g of red mercury(II) oxide powder is placed in an open flask and heated until it is converted to liquid mercury and oxygen gas. The liquid mercury has a mass of 9.26 g. What is the mass of oxygen formed in the reaction? Response ANALYZE THE PROBLEM You are given the mass of a reactant and the mass of one of the products in a chemical reaction. According to the law of mass conservation, the total mass of the products must equal the total mass of the reactants. SOLVE FOR THE UNKNOWN • State the law of conservation of mass. Massreactants = Massproducts mmercury(II) oxide = mmercury + moxygen • Solve for m oxygen. moxygen = mmercury(II) oxide − mmercury • Substitute mmercury(II) oxide = 10.00 g and mmercury = 9.26 g. moxygen = 10.00 g − 9.26 g moxygen = 0.74 g Section Title Essential Questions • What is a physical change and what are several common examples? • What defines a chemical change? How can you recognize a chemical change? • How does the law of conservation of mass apply to chemical reactions? Changes in Matter Section 3 Mixtures of Matter Section 3: Mixtures of Matter Most everyday matter occurs as mixtures—combinations of two or more substances. K What I Know W What I Want to Find Out L What I Learned Mixtures A mixture is a combination of two or more pure substances in which each pure substance retains its individual chemical properties. A homogenous mixture is a mixture where the composition is constant throughout. Homogeneous mixtures are also called solutions. A heterogeneous mixture is a mixture where the individual substances remain distinct. Mixtures of Matter Separating Mixtures Filtration is a technique that uses a porous barrier to separate a solid from a liquid in a heterogeneous mixture. Distillation is a separation technique for homogeneous mixtures that is based on the differences in boiling points of substances. Crystallization is a separation technique for homogenous mixtures that results in the formation of pure solid particles from a solution containing the dissolved substance. Mixtures of Matter Separating Mixtures Sublimation is the process of a solid changing directly to a gas, which can be used to separate mixtures of solids when one sublimates and the other does not. Chromatography is a technique that separates the components of a mixture on the basis of tendency of each to travel across the surface of another material. Mixtures of Matter Essential Questions • How do mixtures and substances differ? • Why are some mixtures classified as homogeneous, while others are classified as heterogeneous? • What are several techniques used to separate mixtures? Mixtures of Matter Section 4 Elements and Compounds Section 4: Elements and Compounds A compound is a combination of two or more elements. K What I Know W What I Want to Find Out L What I Learned Elements An element is a pure substance that cannot be separated into simpler substances by physical or chemical means. • 92 elements occur naturally on Earth. • Each element has a unique name and a one, two, or three-letter symbol. • The periodic table organizes the elements into a grid of horizontal rows called periods and vertical columns called groups. • Elements in the same group have similar chemical and physical properties. • The table is called periodic because the pattern of similar properties repeats from period to period. Elements and Compounds Compounds A compound is a made up of two or more elements combined chemically. • Most of the matter in the universe exists as compounds. • Table salt, NaCl, and water, H2O, are compounds. • Unlike elements, compounds can be broken into smaller components by chemical means. Elements and Compounds Compounds Separating a compounds into its elements often requires external energy, such as heat or electricity. This figure shows electrolysis of water to form hydrogen and oxygen gas. Elements and Compounds Law of Definite Proportions The law of definite proportions states that a compound is always composed of the same elements in the same proportion by mass, no matter how large or small the sample. Example: Water is always composed of 2 Hydrogen to 1 Oxygen The relative amounts are expressed as percent by mass, the ratio of the mass of each element to the total mass of the compound expressed as a percentage. Elements and Compounds Law of Definite Proportions This table demonstrates that the percentages of elements in sucrose remain the same despite differences in sample amount. Elements and Compounds Law of Multiple Proportions The law of multiple proportions states that when different compounds are formed by a combination of the same elements, different masses of one element combine with the same relative mass of the other element in whole number ratios. Example: Peroxide, H2O2, and water, H2O • • Different compounds formed from the same elements. Hydrogen mass the same in both compounds but oxygen mass is a 2:1 ratio in peroxide to water. Elements and Compounds Law of Multiple Proportions Elements and Compounds Essential Questions • What distinguishes elements from compounds? • How is the periodic table organized? • What are the laws of definite and multiple proportions and why are they important? Elements and Compounds