Barometry
advertisement

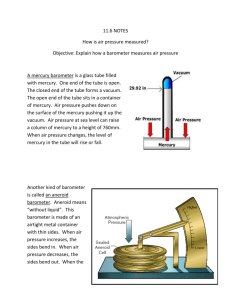
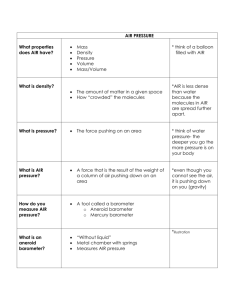
Barometry The art or science of barometric observation History • Giovanni Batista Baliani – observed that syphon pumps could not pump water higher than ~ 34 feet • Galileo - proposed it was due to a vacuum • Gasparo Berti – created the first working barometer sometime between 1640 and 1643 • Evangelista Torricelli – credited with inventing the barometer in 1643 Atmospheric Pressure • The atmosphere exerts a pressure on the surface of the Earth equal to the weight of a vertical column of air Static Pressure Calculation • To calculate static pressure (p) at the surface, use: p = ρ(z)g(z)h • ρ(z) = air density at altitude (height) of measurement • g(z) = gravitational acceleration at altitude of measurement • h = height above sea level Units of Pressure • • • • • SI unit of pressure is the pascal (Pa) kg m-1 s-2 Preferred unit in meteorology is the millibar 1 mb = 1hPa = ___Pa 1 in. Hg (@ 273.15 K) = _____ hPa 1 standard atmosphere (sea level) = ______hPa = _______ mb Three Types of Pressure Measurements • Absolute – total static pressure (ie barometric pressure) • Gauge – pressure relative to ambient atmospheric pressure • Differential – pressure relative to some other pressure Static vs Dynamic • Static pressure is actual air pressure • Dynamic pressure is pressure exerted by wind flow • Dynamic pressure can produce errors in static pressure measurement Direct (In-situ) Measurement of Pressure • Balancing the force of the atmosphere against the weight of a column of fluid (Fortin barometer) • Balancing the force of the atmosphere against the force of a spring (Aneroid barometer) Mercury Barometers • Manometer is the simplest form of a mercury barometer • Can be either open-ended (Differential pressure measurement) or close-ended (Absolute pressure measurement) • Can be awkward to use since manual measurements of fluid height in each arm and the difference calculated to get the raw output Fortin Barometer • Improved version of the manometer offering high accuracy and easy calibration. Somewhat portable. Excellent long-term stability • A column of mercury is enclosed in a glass tube that is sealed at the top with a reservoir of mercury at the bottom. • A vacuum is created at the top of the tube Fortin Barometer • Height of the column of mercury is determined using the attached scale • The height of the mercury in the reservoir must be adjusted upwards using an adjusting screw to the fiducial (reference) point • The measurement is then taken from the attached scale Fortin Barometer Measurements • Open the case and immediately read the temperature • Use the bottom screw to adjust the height of the mercury to the fiducial point. • Adjust the scale index to the top of the mercury column. Keep your eye level with the mercury meniscus in the tube. • Read the pressure using the vernier • Lower the level of the mercury in the cistern Why Use Mercury? • Has a high density (14x heavier than water) leading to a column that is of reasonable length • Low vapor pressure • Easily purified and chemically stable • Remains liquid for a wide range of temperatures (-38.87C – 356.58C) Sources of Error for Mercury Barometers • Dynamic wind pressure can produce (positive and negative) errors on the static measurement on the order of several millibars • Density of mercury is a function of temperature so temperature effects must be compensated for Sources of Error for Mercury Barometers • Since the force of the atmosphere is balanced against the weight of the mercury in a column, local gravity must be known and a gravitational correction calculated • The presence of gas (other than mercury vapors) in the vacuum portion of the tube will cause an error in measurement Sources of Error for Mercury Barometers • The surface tension of mercury will cause a depression in the mercury column of smallerbore tubes. The correction for this is usually incorporated into the index correction (calibration). • The barometer must be kept vertical • Impurities affect the density and measurement Temperature Correction • The temperature correction for a Fortin barometer is CT = _________ • β= Volume and Linear Expansion Coefficients • • • • • Mercury (β): 1.818 x 10-4 K-1 Aluminum (α): 23.0 x 10-6 K-1 Brass (α): 18.9 x 10-6 K-1 Steel (α): 13.2 x 10-6 K-1 Iron (α): 11.4 x 10-6 K-1 Calculating Local Gravity • Start by calculating gravity at sea level at the barometer latitude, ϕ • gϕ = Calculating Local Gravity • Then calculate the elevation effect • gL = Correcting for Local Gravity • The correction factor for local gravity is given as: • CG = Corrected Station Pressure • The raw barometer reading is converted to station pressure by: • ps = Aneroid Barometers • Consists of an evacuated (vacuum) chamber with a flexible diaphragm that moves in response to an applied pressure. The restoring force is a spring that may be part of the diaphragm • Aneroid – “without fluid” • Two types – Metallic-diaphragm and silicondiaphragm Calibration Equation for Aneroid Barometers • p= Bourdon Aneroid Barometer • Consists of a flattened tube with round ends bent in a circular pattern. The tube is open to the ambient pressure but is enclosed in an evacuated box. • As pressure increases, the tube tries to assume a circular form, causing it to straighten out. This movement can be correlated to pressure. Sources of Error for Aneroid Barometers • • • • Exposure Errors Temperature-induced errors Hysteresis Effects Drift Indirect Measurement of Pressure • Indirect – measurement of a variable other than pressure that is a function of pressure • Boiling point of a liquid – Boiling point of pure water at standard sea-level pressure is 373.15 K – Decreases with increasing height Hypsometer • Height Meter • Contains a flask of fluid, heated to maintain continuous boiling with a temperature sensor • Need to know the relationship between vapor pressure and temperature to derive pressure Vapor Pressure • The pressure of a vapor in equilibrium with its non-vapor phases • Dependent on temperature • Related through the Clausius-Clapeyron Equation Hypsometer Pressure Calculation • p= Comparison of Barometer Types • Mercury Barometers (Fortin) – – – – – – – Simple physical concept Require no calibration Difficult to automate and transport Must be kept vertical Needs temperature and gravity corrections Mercury vapor is toxic Improper handling may introduce bubbles into mercury column – Height of column cannot be changed Comparison of Barometer Types • Aneroid Barometers – – – – – – – Very small size, easily portable Easily automated Insensitive to orientation, motion and shock No gravity correction needed No toxic chemicals Concept is simple, but calibration is always required Temperature sensitivity is high, no simple or predictable correction – Subject to unpredictable drift Comparison of Barometer Types • Hypsometer – Small size, reasonable portable – Easily automated – Sensitive to orientation – No gravity or temperature correction needed – No drift or hysteresis – Concept is simple, no calibration required Exposure Error • Barometers are designed to measure static pressure • Need to be isolated from dynamic effects • Impractical for barometers to be inside buildings unless equipped with a static port • Static port needs to extend beyond the pressure field of the building Exposure Error • Pressure field of building can be 2.5x building height vertically and 10x height of the building horizontally • Still need static port for mounting outside on towers • Vertical static port still doesn’t completely reduce the dynamic error, but keeps it to a minimum as long as the static port is kept vertical Exposure Error • Can be very problematic for pressure measurements on buoys