6a Lab Instructions
advertisement

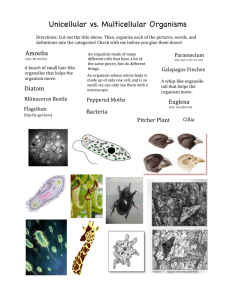
Lab 6 Instructions EVALUATE LAST WEEK’S MATERIALS Chapter 45: Skin Bacteria Each INDIVIDUAL has one Mannitol Salt Agar (MSA) plate inoculated with a swab from your own skin surface left over from the last lab period before the break. Use a needle to touch one colony from the MSA plate and make a suspension from the colony in a small volume of water or saline, and then fill the wells with the suspension. Alternatively, the lab tech might have a pure culture of a Gram positive organism ready to inoculate, in which case, you could use a loop instead of a needle. Either way, use a disposable plastic pipette to place a drop of the pure organism suspension in each well. The last two wells on the right need some mineral oil on top. Fill the bubbled plastic case with just enough water to cover the little ice-cube shaped depressions in the bottom; this will humidify the strip. After you inoculate each well, place the whole strip on top of the “ice-cube depressions” in the plastic case. Cover the whole thing with the smooth plastic case cover. Incubate at 37°C. Dispose of the glass vials by placing them in the sharps container. Next week, we will add reagents to some of the wells, and identify which Staph organism it is. This is what all positive tests look like on the API Staph strip This is what all negative tests look like For the API Staph results you will need to use this website to access the key: https://apiweb.biomerieux.com/servlet/Authenticate?action=prepareLogin Login: abarral PW: nubio203a SOIL PROJECT If you have not done so already, use your pure culture to perform the following tests for morphological characteristics Use your pure culture to perform the following tests for cultural characteristics 1) Inoculate a broth to observe colony morphology at the next lab period. 2) Determine if your organism displays hemolysis by inoculating a blood agar plate. Label it with “soil” and the other data so it does not get mixed up with your body swab on blood agar. Use your pure culture to perform the following tests for morphological characteristics 1) Gram stain to determine Gram +/- and shape (rods or cocci) 2) Negative stain to determine shape and arrangements (staph, strep, diplo, singles) 3) Motility stab (p.1 of “How to ID an Unknown” document) 1 4) Hanging drop to observe if it runs or tumbles (p.2 of “How to ID Unknown” document. I also have an instructional video posted on my website) Use your pure culture to perform the following tests for cultural characteristics 2) Inoculate a nutrient broth today to observe colony morphology in a liquid next lab period. 3) Determine the optimal temperature by inoculating 3 TSB tubes, labeled with three different temperatures you want to incubate at (refrigerator, room temp, and incubator). Use the spectrophotometer to calculate their optical density at the next lab period. The tube with the most growth (highest OD) is the temperature they prefer. Use three tubes for each soil colony that had a zone of clearing. Use three tubes for each different colony on you home experiment. 4) Determine the oxygen requirements by inoculating a thioglycolate tube. Next lab period, record if it is a strict aerobe, strict anaerobe, or facultative anaerobe. Do this for each soil colony that had a zone of clearing. Do it again for each different colony on your home experiment. 5) Determine if your organism displays hemolysis by inoculating a blood agar plate. Label it with “soil” and the other data so it does not get mixed up with your body swab on blood agar. Do this for each soil colony that had a zone of clearing. Do it again for each different colony on your home experiment. Use your pure culture to perform some of the following tests for physiological characteristics. Check Bergey’s Manual to see which tests to run to determine the genus (and maybe species) of your organism. Instructions on how to do these tests are in the “How to ID an Unknown” document. The 4 tests below in bold are REQUIRED on your soil bug. The rest are optional. 1) 2) 3) 4) 5) 6) Motility TSB (temperature requirements) Thio broth (O2 requirements) Lactase (MacConkey’s agar) Fermentation broths IMVIC a. Indole test b. MR-VP test c. Citrate 7) Amylase 8) Oxidation-fermentation (O-F) test for glucose 9) Urease broth or slant 10) Gelatinase 11) Phenylalanine deaminase test 12) Sim media 13) Decarboxylase broths 14) TSI (triple sugar iron agar) 15) Catalase 16) Oxidase 17) Lipase 18) Nitrate reduction test NEW LAB CHAPTER WORK: Summary I will demonstrate inoculations today to remind you about sterile technique, then I will watch to see that you are using proper technique. You will inoculate 7 tubes, one slant and one plate with your unknown organism that you will be assigned today. For the MacConkey plate and all the tubes, use a loop For the motility tube, use a needle. Then inoculate another tube with your assigned pure culture for a control. Then observe live protozoa cultures: Euglena, paramecium, and amoeba. Then observe preserved slides of worms. 2 UNKNOWN ORGANISM PROJECT Each of the 8 lab groups will get one of the following organisms, but you won’t know what you have. You need to run some tests on them to find out what you have. Pseudomonas aeruginosa E. coli Proteus vulgaris Enterobacter aerogenes The six tests you will run on your unknown organism today are these: Use a loop for all of them except motility Label each of the below media with “Magrann”, date, UNK # ___ (use your assigned number) On the TSB tubes, ALSO label “TSB” and either “cold”, “room”, or “incubate” 1) Motility Test (use a needle) is labeled SIM (or MIO if is a purple tube). Inoculate straight into the tube, to the bottom, and straight back out. THIS TUBE STAYS AT ROOM TEMPERATURE. 2) Temperature Requirements (3 TSB broth tubes incubated at different temperatures: fridge, room temp, and incubator) 3) Ch. 19: Thioglycolate broth (Thio) for oxygen requirements (don’t shake the broth; stab with a loop straight down into the liquid and back up). Inoculate your soil bug too. 4) MRVP broth (each group inoculate 2 tubes) Inoculate your soil bug too. 5) MaConkey’s agar plate (to detect lactase) Use a loop to make a loose zig zag from top to bottom. 6) Simmons Citrate agar slant (use a loop to zig-zag across the top of the slant). Inoculate your soil bug too. Do not tighten the caps on any of these above tubes; leave them loose Just flame your loop once and do all the inoculations with your unknown organism. Then flame your needle and inoculate the motility tube. Then flame your loop again and repeat the inoculations with the organism you are assigned below. We will use these for controls so we can see what positive and negative tests look like. Controls Group A inoculate one Thio Broth with Pseudomonas aeruginosa (aerobic) Group B inoculate 2 MRVP tubes with E. coli (+/ -) Group C inoculate 2 MRVP tubes with Enterobacter aerogenes (-/+) Group D inoculate 2 MRVP tubes with Proteus vulgaris Label each of the controls with “Magrann”, date, “Control” and the name of the organism Next, observe the following live cultures. Each ROW of students get 3 square plastic DEPRESSION SLIDES in the front of the room by the sink. Carefully use your fingernail to remove the lid from the surface that is not flat. Use a plastic pipette to obtain one of the organisms in the BOTTOM of their jar. Place the drop into the slide, put the cover on, turn it upside down with the flat surface facing up, and observe the organism and its type of motility under a microscope. If it runs too fast, add a drop of Protoslow. Repeat for the other 2 organisms; let everyone in your row see. DO NOT DISPOSE OF THE DEPRESSION SLIDES. Clean them carefully with alcohol, dry them, and put them back. Ch 35 (Protozoa; P. 271 in lab manual) Live protozoa cultures: Amoeba Paramecium Euglena Preserved protozoa slides Giardia (food poisoning from lake water) Trypanosoma (sleeping sickness) Plasmodium (malaria) 3 NEXT TUESDAY BEFORE DINNER BREAK PCR Machine Look at your streak for isolation plate of your soil organism (or your pure culture slant if you have one). For each organism that showed a zone of clearing against anything, give that organism a name that is not a number, such as “Baby”, “Poppy”, Radman”, etc. Once you name each organism you have, go and write your organism name next to one of the list of numbers on the front board so we know how many PCR tubes to prepare. 1. 2. 3. 4. 5. Baby Poppy Radman ….. ….. I will then run the organisms through the PCR machine, which should be done at 8:30 pm, after which, I will take the tubes out, put them in the rack, and place them in the little sub-zero freezer in the back of the room. Ch 14 (Fermentation): We have to inoculate these before the dinner break on the next Tuesday. After we run the soil bugs through the PCR machine, use your unknown organism to inoculate the following tubes and place them in the incubator to examine on Thursday. Flame your loop and do all of the inoculations with your unknown organism. But flame the neck of each tube before and after the inoculation. Do the same with each of your soil organisms (get three more tubes for each organism you ran through the PCR). Write your group # AND the organism # on each tube 1) Dextrose fermentation tube 2) Lactose fermentation tube 3) Sucrose fermentation tube 4