Ozone Metabolism Dr Les Waymack in JD Norris Vet wkshp
advertisement

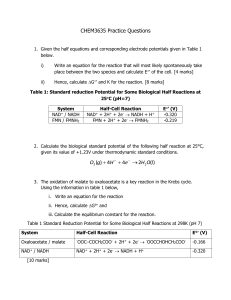
OZONE THERAPY The Role of Oxygen in Metabolism BIOCHEMISTRY IS ORGANIC CHEMISTRY Occurs in living tissues and with a more finite array of reactions. A few of those to be addressed here: Acid + Alcohol -------------------------------> Ester + HOH HYDROLYSIS = Condensation = combining two reactants and excluding HOH Transamination = transferring and amine (-NH2) from one carbon chain to another Deamination = removal of the -NH2 from a carbon chain REDOX = Oxidation-Reduction (very important in cellular respiration) De-carboxylation = removal of CO2 from the carboxyl group of an organic acid “splitting via water” GUIDE TO REACTION SYMBOLS Hydrolysis Condensation (dehydration) Carboxylation De-carboxylation Oxidation Reduction THE SPEED (AND/OR) DIRECTION OF A BIOLOGICAL REACTION may be influenced by any one, or a combination, of the following: Temperature pH (H+ conc.) Enzymes involved Metabolic requirement of the cell Concentration of substrate Nature of the SUBSTRATE and REACTANTS TERMINOLOGY AND SYNONYMS: EMDEN-MEYERHOFF = EM = GLYCOLYSIS (occurs in the cytosol) Reducing agent is oxidized KREBS CYCLE = CITRIC ACID CYCLE = Tricarboxylic acid (TCA) cycle (occurs in the matrix of the mitochondria) ELECTRON TRANSPORT SYSTEM = ETS (occurs in the inner-mitochondrial membrane) KEEP IN MIND: Glycolysis occurs in the cytosol TCA occurs in the mitochondrial matrix Overall process (RESPIRATION) is influenced by: Availability of substrate Metabolic requirements of cell (Demands of the TCA) AVAILABILITY OF OXYGEN GLYCEROL, FATTY ACIDS, AND TRIGLYCERIDES ESTERIFICATION REACTION KETOSIS Accumulation of KETONE BODIES as a result of incomplete fat metabolism and oxygen deficit. The Primary KETONE BODIES associated with KETOSIS: Acetone Acetoacetic Acid Beta Hydroxyl Butyric Acid (ketogenic) REDOX REACTIONS ALWAYS PROCEED TOGETHER: Oxidizing agent is reduced Reducing agent is oxidized VARIOUS EXPRESSIONS OF OXIDATION AND REDUCTION OXIDATION Loss of electrons Addition of oxygen Gain of Protons Loss of H+ ions REDUCTION Gain of electrons Loss of oxygen Loss of Protons Gain of H+ ions REDOX EXAMPLE Oxidation of an alcohol to an acid and the reduction of an acid to an alcohol. (Aldehydes are intermediate products of the reaction) TCA - FUNCTIONS IN THE MITOCHONDRIAL MATRIX CO2 diffuses out as a waste H+ diffuses to the inner-membrane space Electrons (e- ) passed along ETS to molecular oxygen ACETYL COA ENTERS THE TCA AND OXIDIZED COMPLETELY CO2 - diffuses out Electrons - ETS H+ - innermembrane space WHEN THE ENERGY DEMANDS FOR WORK BECOME SO GREAT THAT THE OXYGEN SUPPLY CANNOT KEEP PACE: NADH is “loaded” and has no place to “un-load” (H+ accumulate) Buffer systems can regulate pH up to a point Eventually the ANAEROBIC THRESHHOLD will be reached From this point the body goes into OXYGEN-DEBT (pyruvate becomes an H+ acceptor) THE OXIDATION OF LACTIC ACID AND REDUCTION OF PYRUVIC ACID IONIZATION OF AN ORGANIC ACID WHEN THE CAPACITY TO FORM LACTATE (IONIZED FORM OF LACTIC) IS EXCEEDED: Buffer system overwhelmed H+ accumulate Enzyme systems cease ----- outside their optimum pH NAD+ is reduced to NADH (has no electrical charge) NAD+ (“pack mule”) becomes limiting “PACK MULES” (NAD+ NADP+ FAD+) transport cargo (H+) to the ETS They must “unload” to remain useful to the NAD+/NADH ratio NOW -- ONCE WORK ABATES AND OXYGEN BECOMES AVAILABLE Liver converts lactic back to pyruvic (oxidation) NADH can unload and become available to “reload” H+ accumulation is reversed pH drop is averted (acidosis avoided) ATP formed from ADP Energy is conserved IF OXYGEN IS AVAILABLE (BY WHATEVER MEANS): Pyruvate enters the mitochondria and is decarboxylated Acetate (2-C) is picked up by CoAsH to form Acetyl-CoA then to TCA ELECTRON TRANSFER SYSTEM (ETS) Designed to SLOW THE FALL of electrons (e-) to oxygen via SEVERAL Redox reactions rather than ONE VIOLENT REACTION THE ROLE OF PEROXIDASES IN THE ETS (Preventing the accumulation of hydrogen peroxide) ADENOSINE TRI-PHOSPHATE (ATP) FORMATION OF AN ACID ANHYDRIDE BOND INCREASING THE AVAILABILITY OF OXYGEN: Accelerates the regeneration of NAD+ (therefore recovery time) Regeneration of ATP Multitude of anabolic effects SUMMARY AND CONCLUSIONS: Based on the foregoing discussion ---- it seems logical to assume that— Any mechanism which can enhance the availability of OXYGEN to the cell would: Delay the ANAEROBIC THRESHHOLD and the point of oxygen debt Reduce the stress on the cells BUFFER SYSTEM Reduce the wasteful loss of energy from the initial dietary source of carbon and hydrogen Maintain the availability of NAD+ and therefore, improve the NAD+ / NADH ratio Enhance the healing and recovery process in cases of injury or illness