800 - Paint Valley Schools
advertisement

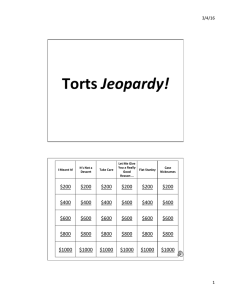
Composition of Matter Energy Water pH Carbon Compounds Molecules of Life $200 $200 $200 $200 $200 $200 $400 $400 $400 $400 $400 $400 $600 $600 $600 $600 $600 $600 $800 $800 $800 $800 $800 $800 $1000 $1000 $1000 $1000 $1000 $1000 Category 1 Category 2 Category 3 Category 4 Category 5 Category 6 $400 $400 $400 $400 $400 $400 $800 $800 $800 $800 $800 $800 $1200 $1200 $1200 $1200 $1200 $1200 $1600 $1600 $1600 $1600 $1600 $1600 $2000 $2000 $2000 $2000 $2000 $2000 Final Jeopardy Scientists Tools Final Jeopardy What are through filtration and evaporation? Name two ways you could separate salt from water in a solution of salt water. $200 What is matter? This comes in 4 different forms and each has mass and occupies space. $400 What is an atom? The smallest particle of matter. $600 What are compounds and an element? CO2 and CO are both classified as this but C by itself is classified as this. $800 What is ionic bonding involves a transfer of electrons whereas covalent bonding involves a sharing of electrons? This is the difference between ionic and covalent bonding. $1000 What is p+ = 2, e- = 2, and n0 = 2 and the atomic number is the top number (number of protons) and the mass number is the bottom number (sum of protons and neutrons)? Determine the number of protons, electrons, and neutrons for Helium. Identify the atomic number and mass number for Helium given the following information. 2 He Helium 4.002602 $200 What is radiant energy and chemical energy? These two forms of energy explain two of the most important energy transformations that occur in biological systems. $400 What is metabolism? These are all of the chemical reactions that take place in an organism. $600 What is the reactants are 4 Fe and 3 O2 and the product is 2 Fe2O3? These are the reactants and products of the following chemical reaction. 4 Fe + 3 O2 2 Fe2O3 $800 What is the activation energy? This is the little (or big) push of energy needed to get a chemical reaction going. $1000 What is an enzyme? A type of protein or RNA molecule found in a living thing that speeds up metabolic reactions without being used up or destroyed. $200 What is hydrogen bonding? This is the type of bonding that water molecules have due to their bond polarity. It allows water to be cohesive, be adhesive, have a high heat capacity, and dissolve many other substances. $400 What is a solute? The substance that gets dissolved in a solution. $600 What is water? This substance is considered the universal solvent because most things (polar substances) will dissolve in it. $800 What is cohesion and surface tension? These properties of water allows water to travel from root to leaves in a plant and some insects to walk on water. $1000 What is less? Hydrogen bonding allows the density of solid ice to be ________ than that of its liquid form. $200 What are bases? The term alkalinity refers to these. $400 What is pH? It literally means “potential hydrogen.” $600 What is an acid? The properties include: gives off H+ in solution, tastes sour, can be corrosive to metals, and turn blue litmus red. $800 What is gives off OH- ions in solution, taste bitter, and turns red litmus blue? These are three properties of a basic substance. $1000 What are buffers? Our bodies have these specific types of chemicals that maintain homeostasis in terms of pH by neutralizing acids and bases. $200 What are organic compounds? Compounds that are primarily made of carbon. $400 What is a monomer? A simple molecule that can combine with other molecules to build bigger molecules. $600 What are polymers? The GAK and slime that we made last year are examples of these and gummy bears too. $800 What are macromolecules? A very large organic compound composed of hundreds or thousands of atoms. They include carbohydrates, proteins, lipids, and nucleic acids. $1000 What is ATP? Energy currency $$$$ $200 What is a monosaccharide? A type of simple sugar (carb) that contains carbon, hydrogen, and oxygen in a 1:2:1 ratio. An example is glucose. $400 What is an amino acid? This monomer unit of a protein that is composed of carbon, hydrogen, oxygen, and nitrogen. They are the building blocks of proteins. $600 What is a polysaccharide? A type of complex sugar (carb) that is composed of three or more monosaccharides. An example is glycogen. $800 What are lipids? Examples of this type of macromolecule include triglycerides, phospholipids, waxes, and steroids. $1000 What is DNA is a double helix whereas RNA is only single stranded? This is the major structural difference between DNA and RNA. $400 answer question $800 answer question $1200 answer question $1600 answer question $2000 answer question $400 answer question $1200 answer question $800 answer question $1600 answer question $2000 answer question $400 answer question $800 answer question $1200 answer question $1600 answer question $2000 answer question $400 answer question $800 answer question $1200 answer question $1600 answer question $2000 answer question $400 answer question $800 answer question $1200 answer question $1600 answer question $2000 answer question $400 answer question $800 answer question $1200 answer question $1600 answer question $2000 answer question Daily Double answer question Daily Double answer question Daily Double answer question The Jeopardy champion!