Chapter 11
advertisement

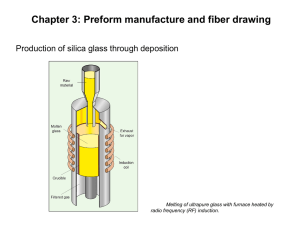
11. Optical Fiber Technology Optical fiber is used worldwide for transmission of voice, data, and content because of its ability to transmit at speeds in excess of 10 GB/second over very long distances. Optical fibers consist of: 1. A core, having high refractive index. 2. Cladding. 3. Buffer, protective polymer layer. 4. Jacket, protective polymer layer. Two methods to manufacture optical glass fiber: 1. Draw the fiber from molten glasses, which are placed in two concentric crucibles (Double Crucible method) 2. Draw from a glass rod called preform. Nowadays most optical fibers are made from the preform. There are three steps in this method: 1. Fabrication of the preform 2. Drawing the fiber from the preform 3. Coating and jacketing process Preform Fabrication: Chemical Vapor Deposition (CVD) 1. Modified Chemical Vapor Deposition (MCVD) 2. Plasma Modified Chemical Vapor Deposition (PMCVD) 3. Plasma Chemical Vapor Deposition (PCVD) 4. Outside Vapor Deposition (OVD) 5. Vapor-phase Axial Deposition (AVD) Chemical Vapor Deposition (CVD) 1. All these methods are based on thermal chemical vapor reaction that forms oxides 2. They are deposited as layers of glass particles called soot 3. Starting materials are solutions of SiCl4, GeCl4, POCl3, and gaseous BCl3. 4. These liquids are evaporated within oxygen stream and form silica and other oxides. 5. Chemical reactions proceed as follows: SiCl4 + O2 -> SiO2 + 2 Cl2 GeCl4 + O2 -> GeO2 + 2 Cl2 4 POCl3 + 3 O2 -> 2 P2O5 + 6 Cl2 4 BCl3 + 3 O2 -> 2 B2O3 + 6 Cl2 - Germanium dioxide and phosphorus pentoxide increase the refractive index of glass - Boron oxide decreases the refractive index of glass. - These oxides are known as dopands. - Changing composition of the mixture during the process influences refractive index profile of the preform CVD Chemical Vapor Deposition is chemical reactions which transform gaseous molecules, called precursor, into a solid material or powder, on the surface of a substrate , in the form of thin film . Vaporization and Transport of Precursor Molecules into Reactor Diffusion of Precursor Molecules to the Surface Adsorption of Precursor Molecules to Surface Decomposition of Precursor and Incorporation into Solid Films Applications may be found in the areas of: Integrated circuits, optoelectronic devices and sensors micromachines, and fine metal and ceramic powders Nanothnic protective coatings - Titanium Carbide-Coated Graphite CVD techniques can be employed to grow a thin layer of protecting materials Modified Chemical Vapor Deposition (MCVD) MCVD Process 1. This method was developed by Bell Laboratories. 2. The gaseous mixture of reactants is fed at the end of a rotating silica tube. 3. This tube is heated by a traversing oxygen-hydrogen burner. 4. As a result of chemical reactions glass particles, called soot, are formed. 5. These particles are deposited on internal wall of the tube. 6. The soot is then vitrified by the traversing burner to provide a thin glass layer. 7. The process is repeated many times as the cladding layers and core layers are formed. 8. When the deposition is finished, the temperature of the burner is increased to collapse the tube into a solid preform. 9. The entire process is highly automated and all process parameters are precisely controlled. Plasma Modified Chemical Vapor Deposition (PMCVD) A modification of MCVD method is a process known as PMCVD. In addition to the normal MCVD technique the radio-frequency coil around the tube generates an internal high temperature plasma. Plasma Chemical Vapor Deposition (PCVD) The PCVD method is similar to PMCVD. The radio-frequency coil is replaced by a microwave cavity resonator. In this method reactions lead directly to of glass layer without forming the soot. Outside Vapor Deposition (OVD) OVD Process 1. This process is also called the “soot process”. 2. It was exclusively used by Corning since the 1970s, and the patent of such a technology has expired since July 2000. 3. Halogens and O2 react in a hot flame to form hot glass soot, which is deposited layer by layer on an aluminium oxide or graphite mandrel. 4. The central mandrel is removed after deposition. 5. In the last step, called sintering, a hollow porous preform is dehydrated and collapsed in controlled atmosphere, (e. g. helium) to form desired preform. Vapor-phase Axial Deposition (VAD) VAD Process 1. In VAD method, the preform can be fabricated continuously. 2. Starting chemicals are carried from the bottom into oxygen-hydrogen burner flame to produce glass soot which is deposited on the end of a rotating silica rod. 3. A porous preform is then grown in the axial direction. 4. The starting rod is pulled upward and rotated in the same way as that used to grow single crystals. 5. Finally the preform is dehydrated and vitrified in ring heaters. 6. This process is preferred for the mass production. Fiber Drawing 1. Optical fibers are obtained by drawing from the preform at high temperature. 2. The drawing process must be integrated with the coating process to avoid contamination of fiber surface. 3. The tip of the perform is heated in a furnace to a molten state. 4. Formed molten gob falls down under the force of gravity while shrinking in diameter into a proper diameter strand. 5. It is controlled continuously during the drawing process. 6. Diameter drift cannot exceed 0.1%. 7. The strand is threaded through a series coating applicators immediately after drawing. 8. Liquid prepolymer coatings are cured by thermal or ultraviolet apparatus. 9. Dual coating, soft inner and hard outer, is needed to avoid microbending and protect against impact and crushing forces in either manufacturing process or installation. 10. Silicone coating and acrylate, Tefzel (ETFE), Teflon (PFA), nylon buffers are applied during the fiber drawing, while additional materials such as Hyrtel and PVC can be extruded after the draw process. 11. The fiber with coatings is pulled down and wound on a winding drum. 12. The drawing process must take place in air conditioned room, because air pollution influences fiber attenuation.