History of Atoms - 18-152
advertisement

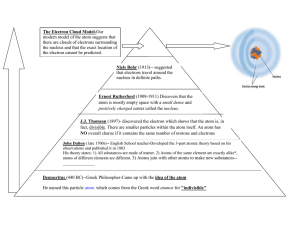
What is an Atom Atoms are the building blocks that make everything we see, touch, eat, hear or smell (humans, chairs, pencils, apples, ect) It made of 3 different parts, the electron, the proton, and the neutron. The proton and the neutron are attached to each other making the nucleus which is the center of the atom. The electron is a tiny negatively charged particle that is much, much smaller than any atom. It circulates the nucleus. Democritus In 400 BC a Greek philosopher ,Democritus, stated that all matter is made up of atoms. He also stated that atoms are eternal and invisible and so small that they can’t be divided, and they entirely fill up the space they are in. He named them “atomos” meaning indivisible in Greek. John Dalton In the 19th century John Dalton came up with an atomic theory that linked to his experiments and observations. This is a summary of the atomic theory. All matter is made of atoms. Atoms are indivisible and indestructible. All atoms of a given element are identical in mass and properties. Compounds are formed by a combination of two or more different kinds of atoms. A chemical reaction is a rearrangement of atoms. Atoms can be neither created nor destroyed. J.J Thomson In 1897 J.J Thomson changed the view of an atom by discovering the electron. He discovered it while doing an experiment by applying high voltages to gases at low pressure. Some experiments showed that it would take about 2000 electrons to weigh the same as the lightest atom, hydrogen. Since they were so small Thomson suggested that they could only have come from inside atoms. So Dalton's idea of the indestructible atom is not fully true. He said that the tiny negatively charged electrons must be inside a pool of positive charge. He called this the plum pudding. Ernest Rutherford Ernest Rutherford found out that the positive charge must be in a small value in the center of the atom and that the electron orbits around the nucleus. He found this out while doing an experiment with radiation. He came up with a new model and it was different from Thomson’s model. Niels Bohr Niels Bohr stated that the electron orbits the nucleus without losing energy and that they only move in fixed orbits of specific energies. He also said that electrons with low energy would orbit closer to the nucleus while electrons with high energy orbit further from the nucleus. He came up with a new atomic model. Bibliography "History of the Atom." History of the Atom. N.p., n.d. Web. 13 Oct. 2012. <http://web.neo.edu/rjones/Pages/1014new/Lecture/chemistry/chapter_8/pages/hist ory_of_atom.html>. "Chemistry Project : The History Of The Atom." Chemistry Project : The History Of The Atom. N.p., n.d. Web. 13 Oct. 2012. <http://hi.fi.tripod.com/timeline/>. "History of the Atom." History of the Atom. N.p., n.d. Web. 13 Oct. 2012. <http://www.absorblearning.com/chemistry/demo/units/LR301.html>. "HISTORY OF THE ATOM FROM DEMOCRITUS TO BOHR AND SCHRÖDINGER." HISTORY OF THE ATOM FROM DEMOCRITUS TO BOHR AND SCHRÖDINGER. N.p., n.d. Web. 13 Oct. 2012. <http://profmokeur.ca/chemistry/history_of_the_atom.htm>. "Science Is Easier than It Sounds." : What Are Atoms Made Of? N.p., n.d. Web. 13 Oct. 2012. <http://science-is-easy.blogspot.com/2009/11/what-are-atoms-made-of.html>.