Intro to Water Health
advertisement

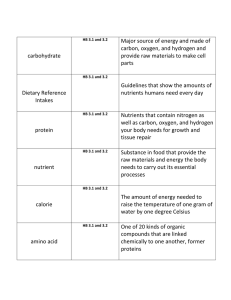
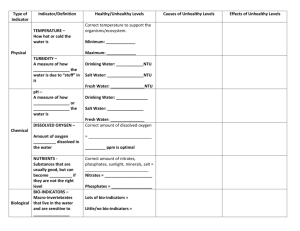
WARM-UP • Update your Table of Contents • Write your homework! • Get a list of Vocab Set 4 off the front table Date Session # 10/23 21 Activity Page # Water Quality Indicator Note guide 36 Water Diagnosis Chart 37 SOMEBODY CALL THE DOCTOR! AN INTRODUCTION TO WATER HEALTH HOW DO WE KNOW IF OUR WATER IS HEALTHY? There are PHYSICAL, CHEMICAL & BIOLOGICAL indicators that work together to determine the health of the water TEMPERATURE • Definition: How hot or cold the water is due to kinetic energy • Healthy:The water is the right temperature to support the things that live in it. • Unhealthy:The temperature is too high ( 970F) or too low (320F) and will hurt or kill things that live in it. TEMPERATURE Causes: • loss or gain of shade • heat pollution • seasonal changes/global changes Effects: • decreased levels of dissolved oxygen (DO) • increased bacteria levels • increased algae growth TURBIDITY • Definition: A measure of how cloudy water is due to “stuff” in it. • Healthy: Clear, clean water = 1 NTU • Unhealthy: Sediment or pollution that makes the water cloudy = 5 NTU TURBIDITY Causes: • erosion • run-off • disturbance of water • algae growth Effects: • increased temperature • decrease in DO • clogs gills in organisms PH • Definition: The acid content of the water • Healthy: Has to be the correct level for the type of water. Most Freshwater: 6.0 8.0 • Unhealthy: Is not the right level for the type of water or to support life. PH Causes: • acid rain • minerals washing into water Effects: • death to sensitive plants and animals PH MINI LAB Levels: 06 is acidic 7 is neutral 814 is basic PH MINI LAB • To demonstrate the idea of pH, you will test 4 things and record their pH level and determine if they are an acid, base or neutral: Lemon Juice pH Value Acid, Base or Neutral? Windex Tap Water Your Mouth DISSOLVED OXYGEN • Definition: oxygen gas dissolved in the water • Healthy: Right amount dissolved oxygen = lots of life 4-5 ppm is optimal • Unhealthy:Low dissolved oxygen = little or no life DISSOLVED OXYGEN Causes: • increased temperature • decaying plant matter Effects: • less diversity of species • fish kills NUTRIENTS • Definition: Something that is usually good, but can become toxic when there is too much • Healthy: The right amount of nitrates, phosphates, sunlight = lots of life • Unhealthy: Too many or not enough nutrients = little or no life • nitrates = above 1 ppm • phosphates = above .003 ppm NUTRIENTS Causes: • fertilizer run-off • animal waste run-off • detergent waste from industry Effects: • EUTROPHICATION • low DO EUTROPHICATION FLOW CHART EXCESS NUTRIENTS ALGAL BLOOM (POND SCUM) DARKNESS BELOW SURFACE PHOTOSYNTHESIS STOPS DECOMPOSERS & OTHER ORGANISMS USE UP OXYGEN DEATH BIO-INDICATORS • Definition: Macro-invertebrates that live in the water and are sensitive to pollution • Healthy: A lot of bio-indicators = healthy water • Unhealthy: Little or no bio-indicators = unhealthy water BIO-INDICATORS Causes: • increased levels of pollution Effects: • damages food chain • loss of organisms “GAME CHANGERS” MERCURY • Natural element that has increased to toxic levels due to industry • Usually affects people through eating fish from mercury contaminated water • Causes kidney damage & brain/neurological disorders “GAME CHANGERS” COLIFORM BACTERIA • commonly found in water, soil and vegetation • usually harmless but can indicate the presence of other harmful forms of coliform bacteria (fecal matter & E. Coli) YOU BE THE DOCTOR! • Use the notes you just took to: 1) Diagnose the possible cause of the water issues on the Water Quality Checklist 2) Decide if the water is healthy or unhealthy VOCABULARY SPLASH • All of the vocabulary words will be on the slide. • Study the words for 30 seconds. • Next slide will be missing 1 of the words from the original slide. • Write down the word that is missing and its definition as quickly as possible. Nutrients pH Nutrients pH Nutrients pH Nutrients pH Nutrients pH Nutrients pH Nutrients pH Nutrients Nutrients pH Nutrients pH HOMEWORK • Vocab Set 4 – Day 1 • Re-send presentations if you want to present on Thursday! • Bring rain boots/shoes you can get dirty for class nextTuesday! WARM-UP • Update your Table of Contents • Write your homework, and get your vocab cards out to be checked Date Session # 10/25 22 Activity CHMS Creek Stewardship Survey Page # 38 CHMS WATERSHED SURVEY: DAY 1 • To prepare for our trip to the creek we need to collect observations about the topography and condition of the CHMS school grounds. (What goes on up here that may be affecting the creek at the bottom of the hill behind the school?) When we get outside, you may bullet point these observations, you may also wish to include sketches or pictures of areas that may impact the creek. • Based on what you have observed, your knowledge of water quality indicators and the way humans impact the hydrosphere...what can you predict is the condition of the creek water? Briefly describe what specific conditions you expect to find and then transfer your predictions to the data table on the back. CHMS WATERSHED SURVEY: DAY 2 • Use your notes and observations to complete the following: • Your hypothesis: (what can you predict the condition of the creek water will be?) “I predict that ___________________ will be at unhealthy levels in the creek because……. • Complete the prediction section of the data table: (what specific conditions do you expect to find?) (+) indicates that you predict levels for that specific indicator will be healthy (-) indicates that you predict levels for that specific indicator will be unhealthy HOMEWORK • Vocab Set 4 – Day 2 • Bring rain boots/shoes you can get dirty and appropriate clothing for class next Tuesday!