Supplements Report Revised
advertisement

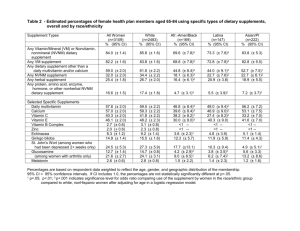
1 Bradon Liddle Julie Roberts English 2010 November 3, 2013 The Truth Behind Dietary Supplements Disgraced baseball star Barry Bonds maintains to this day that he never knowingly took steroids in his life; that the illegal substances he used while playing for the San Francisco Giants were believed to be all natural flax seed oil (“Memorable quotes from Barry Bonds”). While this was hotly debated among sports fans during the scandal, the public has largely sided against Bonds’ claim that he didn’t know he was using performance-enhancing drugs. Bonds was one of the first athletes to make this claim, but he would hardly be the last. While these statements should be taken at with a grain of salt, there is growing validity to them. The sports supplement industry is growing rapidly in the United States and hardly any of these supplements that are being sold over the counter are FDA approved. Nearly anyone can walk into their local GNC or go online to established websites and order the newest supplement being used by body builders and athletes to enhance their workouts without ever really knowing what is in these products. There is very little oversight on this nearly $30 billion-a-year industry (e.g. see chart 1) and manufacturers are trying to stay ahead of the curve and provide the results athletes want, at sometimes severe costs. There are numerous horror stories of teens who 2 have taken these over the counter supplements and had serious health problems and even death, mainly because they believed what were taking was safe. History of the Supplement Industry Some form of “supplements” have been around for hundreds of years, whether they were labeled that or not. In the late 19th century John S. Pemberton, the pharmacist who invented Coca-Cola, first marketed the product not as a soft drink but as a medical treatment to help "ladies, and all those whose sedentary employment causes nervous prostration” (Pendergrast 24). The supplement industry as we know it today is structured the way it is largely due to Dietary Supplement Health And Education Act of 1994. This bill allows any product containing a vitamin, a mineral, an herb or other botanical, an amino acid, a dietary substance for use by man to supplement the diet by increasing the total dietary intake, or a concentrate, metabolite, constituent, extract, or combination of any of the aforementioned ingredients to be labeled a “supplement” (United States Congress). The manufacturer of the product can then include any of the previously listed ingredients in their supplement and make the claim that the product will deliver the result of (weight loss, fat loss, muscle gain, weight gain, etc.) as long as they include the disclaimer "This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease” as the bill exempts these supplements from federal review of their safety or effectiveness before they go on sale (United States Congress). 3 These seemingly contradictory declarations can cause confusion in the consumers of the product. Steven Novella, a clinical neurologist at the Yale School of Medicine, stated “the consequences are we have an effectively unregulated market for these products, a Wild West, and people are being abused by slick marketing, and as a result taking things that are worthless or in some cases not even safe” (Lipton). Since the introduction of the bill the industry has ballooned. In 1994 when the bill was passed there were about 4,000 products on the market compared to some 55,000 today (Lipman). (Chart 1 source: “U.S. nutrition industry grows despite recession” Nutrishop. Web. 13 October 2013) 4 Many critics of the industry point to the lobbying efforts and campaign contributions made by the industry to politicians who push through laws that benefit the industry as the reason these products are so loosely regulated. Utah Senator Orrin Hatch (R), the chief author of the Dietary Supplement Health and Education Act, “has been rewarded with hundreds of thousands of dollars in campaign contributions, political loyalty and corporate sponsorship of his favorite causes back home” from the supplement industry and his own son works as an industry lobbyist in Washington (Lipton). Marc S. Ullman, who works as a lawyer for several different supplement companies, calls Senator Hatch a “natural ally” (Lipton). Hatch however counters that there are provisions in his bill to allow for regulation of the industry, pointing out that the bill requires manufacturers to list all the ingredients used in the product in descending order by weight (United States Congress). Hatch also says he supports the industry so vigorously because he believes in it, using supplements himself. Hatch does concede that sometimes dangerous products are released to market but not because new legislation is needed but because “the F.D.A. doesn’t have the money to really do what it should do” (Lipton). Dangers of Dietary Supplements Because of the murky regulatory waters the supplement industry treads in many dangerous products have been released to market with no safety testing done on the product. Matt Cahill, a giant in the supplement industry, has been producing 5 dietary supplements for over a decade despite serving time in federal prison for past offenses in the supplement industry and facing more charges today (Young). Cahill designed the best selling pre-workout “DS Craze,” which was named the 2012 “New Supplement of the Year” by bodybuilding.com. The product is aimed at athletes to use before a workout or event and promises “endless energy.” The product however has come under criticism as lab tests conducted by the U.S. antidoping agency has yielded results of amphetamine-like compounds in samples of the product that claims to be “all natural” (Young). The product, which has now been pulled from shelves at GNC and online at sites such as Amazon, Walmart, and bodybuilding.com, has come under further scrutiny since popular fitness model Rob Riches blamed a failed drug test on the product. Riches has built his career around being a “drug-free” body builder and recently lost out on the British National Championship due the failed drug test, the first of his career (Young). Riches pointed out that nothing on the labeling or manufactures website has any banned substances listed among the active ingredients (Young). Cahill had also previously produced and marketed a weight loss supplement that contained DNP, a highly toxic chemical used in pesticides, even though the substance had been banned against human use since the 1930s (Young). The product, specifically the DNP, was listed as the cause of death of a 17-year-old girl in 2002 when she overdosed after taking too many of the capsules. Cahill and his partner were not charged with the death of the teenager however as it was believed to be a suicide attempt (Young). "Any industry is going to have some fringe players that don't always follow the rules," said Steve Mister, president of the Council for Responsible Nutrition, also pointing out that 6 nearly 150 million Americans use supplements safely with few reported adverse health effects. Federal regulators, however, claim that these are not fringe elements, that “there are a lot of bad actors” in this industry (Young). The danger in these supplements not only lie that they do not have to be proven safe before going to market like drugs do but that federal regulators have little power and resources to deal with dangerous products. The process the FDA must go through to ban a product is very arduous and as a result the FDA has only ever banned one ingredient used in the supplement industry…Ephedra (Lipman). Daniel Fabricant, director of the Food and Drug Administration's dietary supplements division states that when they believe a product to be dangerous to the public and want to take it off the market regulators “have to show that a product is harmful, is unsafe under all conditions of use, which is a significant scientific burden," adding "So it doesn't happen overnight” (Young). Are There Safe Supplements? While there are many athletes who will gladly accept the risks of these supplements believing that the trade off of adverse health affects is acceptable if it helps them more effectively reach their goals the question remains, is there a way for athletes who take supplements to do so safely? Yes there are avenues for athletes to be safe when using supplements. Dr. Marvin Lipman, Consumer Reports chief medical advisor, suggests using supplements from well known and established manufactures with a positive track record and if you have any reservations about a 7 manufacturer or product research them on trustworthy sites such as Consumerreports.org, FDA.gov, MedlinePlus.Gov, and USP.org (Lipman). Also just because a product is marketed as natural doesn’t mean its safe. Be wary of “natural” supplements, as many have been linked kidney and liver problems and even cancer (Lipman). And most importantly be sure to tell your own personal physician of any supplements you are taking or considering taking, as they have a more personal relationship with you and your medical history. Dr. Lipman also urges consumers to report any side effect you have had with supplements to the FDA by submitting a report online or by calling 1-800-332-1088 as it is estimated that “less than 1 percent of adverse reactions are ever reported” (Lipman). 8 Works Cited “Memorable quotes from Barry Bonds regarding steroids.” USA Today 13 April 2011. Web. 8 October 2013 Lipman, Marvin, M.D. “The Dangers of Dietary Supplements.” Consumer Reports. June 2012. Web. 15 October 2012 Lipton, Eric. “Support Is Mutual for Senator and Utah Industry.” New York Times 20 June 2011. Web. 15 October 15, 2013 Pendergrast, Mark. For God, Country and Coca-Cola: The Unauthorized History of the Great American Soft Drink and the Company That Makes It. Basic Books 2013. Print. United States. Cong. Senate. 103rd Congress, S. 784, Dietary Supplement Health and Education Act of 1994 [introduced in the U.S. Senate; 7 April 1993]. GPO Access. Web. 15 October 2013. Young, Alison. “Sports supplement designer has history of risky products.” USA Today 27 September 2013. Web. 14 October 2013.