HLA-B*5701
advertisement

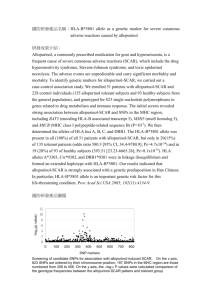
The Role of Pharmacogenetics in Safety Assessment Michael Mosteller, Arlene R. Hughes, Sara H. Hughes, and Matthew R. Nelson 32nd Midwest Biopharmaceutical Statistics Workshop Ball State University, May 19, 2009 Slide 2 What is Pharmacogenetics? Some Basic Genetics Single Nucleotide Polymorphism (SNP) – A single nucleotide polymorphism (SNP) with two sequences that differ at a single position: ATCGC and ATTGC Allele - Any of two or more alternative DNA sequence patterns that occur at a given location in the genome. – For the SNP above, the possible alleles would be: C and T Genotype - The alleles present in a particular person at a particular location in the genome. – For the above SNP, the possible genotypes are: C/C, C/T, or T/T Genetic Markers – Locations in the genome at which people have differing DNA sequences. – Can be used as genetic landmarks (SNPs are an example) Phenotype – A general term that refers to any non-genetic feature of an organism. U.S. Department of Energy Genome Research Programs: genomics.energy.gov Available at: www.ornl.gov/sci/techresources/Human_Genome/publicat/primer2001/primer11.pdf What is Pharmacogenetics? Some Historic Highlights Slide 3 1950’s - Drug responses linked to known genetic deficiencies – G6PD deficiency + primaquine = hemolysis – “Pharmacogenetics” proposed in 1959 1980’s - Dawn of the molecular genetics/genomics era – The number of genetic markers increased dramatically – “Pharmacogenomics” coined in 1986 1990’s - Human Genome Project and the SNP Consortium – More (and more) genetic markers 2000’s - The HapMap Project – Over 3 million SNPs studied in 270 individuals of European, African, Japanese and Chinese ancestry 2000’s - Advances in DNA Microarray Genotyping Chips – Rapid, relatively inexpensive, genome-wide genotyping of ~1 million SNPs with commonly occurring alleles. The stage is set to explore the possibilities of pharmacogenetics. Slide 4 What is Pharmacogenetics? Definition & Essence of a Pharmacogenetic Analysis Pharmacogenetics: The study of the influence of genetics (variations in DNA sequence) on drug response. Statistical Bottom Line: Does a genotype (or allele) have a significant effect on how an individual responds to a drug? % of Patients with Bilirubin Change > 2 mg/dl Enzymes Cell Surface Receptors Antibodies Medicine Response Adverse Reaction Symptoms Abnormal Lab Values …or binary. can The endpoint be continuous… P = 2 x 10-22 6,6 6,6 6,7 UGT1A1 Genotype 6,7 7,7 7,7 (Danoff et al., The Pharmacogenomics Journal (2004) 4, 49–53) UGT1A1 Genotype (rs3064744) (Adapted from Danoff et al., The Pharmacogenomics Journal (2004) 4, 49–53) Slide 5 Drug Safety: Key Role in Personalized Medicine The Vision of Personalized Medicine: – – – – The right medicine to the right patient at the right dose Optimize the benefit/risk ratio for the individual Reducing safety risk is critical Lots of collaborative activity… Slide 6 Drug Safety: A Special Focus for FDA Numerous initiatives to improve drug safety International Serious Adverse Events Consortium (SAEC) – Formed in 2007, with strategic and scientific support from FDA – Initial projects: Phase 1 ― SAEC Membership (11) Spanish DILI Drug induced liver injury Stevens Johnson Syndrome EUDRAGENE – Future projects under consideration include: QT Prolongation Hypersensitivity Rxn Rhabdomyolosis Edema Renal Failure DILIGEN Top 5 SAEs External Collaborators/Contributors SAE Consortium Slide 7 A Safety Pharmacogenetics Success Story HLA-B*5701 status predicts susceptibility to hypersensitivity reaction to abacavir sulfate. Good uptake into clinical practice; referenced in US product label: Steps Leading to Full Clinical Implementation of a Safety Pharmacogenetics Association From Marker Discovery to Clinical Implementation – Discovery of the statistical association – Confirmation of the statistical association – Proposal of a biological hypothesis – Demonstration of clinical utility – Demonstration of generalizability among ethnically diverse patients – Provision of a pharmacogenetic marker test – Demonstration that cost effectiveness is likely – Incorporation of pharmacogenetic information in product label – Education of patients, physicians, regulators, and payers Slide 8 Slide 9 Abacavir Hypersensitivity Reaction Abacavir (ABC): Effective HIV medicine Hypersensitivity reaction (HSR) in 2-9% Symptoms: Fever, rash, malaise, GI symptoms Symptoms resolve with permanent discontinuation Rechallenge can result in a life-threatening or fatal reaction Effective clinical management program developed Pharmacogenetics program initiated to improve benefit/risk – Candidate genes (PK and immune) – Genome Scan Slide 10 Discover An Association CNA30027, a retrospective case/control study in 100 HSR Cases, 200 Controls 2001: Unprecedented results seen in an interim analysis of CNA30027 Association later refined to HLA-B*5701 Based on data in Hetherington, et al., Lancet 2002; 359: 1121–22. Slide 11 Confirm the Association Dr. Simon Mallal was also conducting PGx research on ABC HSR 2001: Mallal et al., independently identified the association between HLA-B*5701 and ABC HSR Therefore, association was seen in two independent sample sets Results from a later report: Western Australian HIV Cohort Study* HSR No HSR B*5701+ 17 4 B*5701- 1 226 Sensitivity 94% Specificity 98% * Martin, et. al, PNAS 2004; 101:4180-4185. Slide 12 Reasonable Biological Hypothesis Biological mechanism not critical for prediction purposes, but… Bolsters evidence provided by other findings Abacavir Aldehyde (ABC Metabolite) B*5701 Naisbitt DJ, Pirmohamed M, Park BK, Current Allergy and Asthma Reports 2003;3:22-29 Slide 13 Show Clinical Utility is Likely No universal criterion for utility One criterion: AE incidence reduction Using retrospective data… Genotype Result ABC Hypersensitivity Present Absent True 17 False 4 B*5701+ Positive Positive False B*5701Negative Total 1 18 True 226 Negative 227 • HSR, screening: 1/227 = 0.4% 230 248 • HSR, no screening: 18/248 = 7.3% What if HLA-B*5701+ patients were screened out? Based on these results Martin et al. (2004) concluded HLA-B*5701 could be useful in reducing HSR incidence Slide 14 Demonstration of Clinical Utility in a Prospective, Randomized Clinical Trial: PREDICT-1 PREDICT-1: Large, prospective GSK clinical study to assess the utility of HLA-B*5701 screening ABC-containing regimen with HSR monitoring according to Standard of Care (~900) ABC Naïve Randomize (1:1) Subjects (~1800) ABC-containing regimen Exclude Subjects who are B*5701 positive Prospective HLA-B*5701 Screening (~900) Compare HSR incidence in Standard of Care Arm and HLA-B*5701 Screening Arm Enroll Subjects who are B*5701 negative Hughes S, Hughes A and Brothers C et al. PREDICT-1 (CNA106030): The first powered, prospective trial of pharmacogenetic screening to reduce drug adverse events. Pharmaceutical Statistics 2007 PREDICT-1: The First Pharmacogenetic Study of its Kind Slide 15 Slide 16 PREDICT-1: Primary Study Endpoints Co-primary endpoints comparisons between study arms of: – Incidence of clinically-suspected ABC HSR – Incidence of immunologically-confirmed ABC HSR Major symptoms of clinically-suspected hypersensitivity Hetherington S, et al. Clin Ther 2001; 23: 1603-14 Slide 17 PREDICT-1 Incorporated Skin Patch Testing: A tool to refine HSR phenotype Adhesive surface 1% abacavir 10% abacavir Petrolatum control Excipient control Research tool used to identify patients with immunemediated ABC HSR Requires prior ABC exposure Phenotype refinement without need for rechallenge Phillips et al. AIDS 2002 and 2005 Phillips et al. IAS 2007 Abstract MOPEB001 24-hour reading (48 hour reading) Slide 18 PREDICT-1: Design & Analysis Details Sample size of 1806 provided ≥90% power to detect – 50% reduction in clinically suspected HSRs – 80% reduction in immunologically confirmed HSRs Power for immunologically confirmed HSR endpoint >99%; power for clinically suspected HSR endpoint = 90% overall study power approx. 90% (>0.99 x 0.90) Closed test approach for analysis of co-primary nested endpoints (immunologically confirmed HSRs are a subset of clinically suspected HSRs) – if significant reduction in immunologically confirmed HSRs then test for reduction in clinically suspected HSR endpoint HSR rates compared between study arms using logistic regression, adjusting for randomisation strata and other prognostic factors Slide 19 PREDICT-1 Key Results: Comparing HSR Rates in PGx Screening Arm versus Standard of Care Arm Prospective Screen Evaluable N=802 Standard of Care Evaluable N=842 0/802 (0%) 23/842 (2.7%) Immunologically Confirmed HSR Statistical Results Exact Odds Ratio[1] (95%CI): 0.03 (0.00, 0.18) P-value: p<0.0001 Clinically Suspected HSR Model Based Proportions [2] Statistical Results Evaluable N=803 Evaluable N=847 27/803 (3.4%) 66/847 (7.8%) 3.3% 7.9% Odds Ratio[1] (95%CI): 0.40 (0.25, 0.62) P-value: p<0.0001 [1] Odds ratio adjusted for actual strata of race, ART status, introduction of NNRTI and concurrent PI use. [2] Model-based proportions calculated using parameter estimates and subject characteristics in the overall population. ART = Antiretroviral therapy NNRTI = Non-nucleoside reverse transcriptase inhibitor PI = Protease inhibitor Slide 20 PREDICT-1 Key Results: Test Characteristics for Predicting Immunologically Confirmed HSR Immunologically Confirmed HSR No Immunologically Confirmed HSR Total HLA-B*5701 Positive 23 25 48 HLA-B*5701 Negative 0 794 794 23 819 842 Total Specificity: Sensitivity: PPV: NPV: 794/819 = 96.9% 23/23 = 100% 23/48 = 47.9% 794/794 = 100% 95% CI (95.5%, 98.0%) 95% CI (85.2%, 100.0%) 95% CI (33.3%, 62.8%) 95% CI (99.5%, 100.0%) Calculated using the Standard of Care arm data only Slide 21 PREDICT-1 Key Results: Covariate Assessment Model Covariates Prospective Screen Arm vs. Standard of Care Arm Immunologically Clinically Confirmed HSR Suspected HSR p < 0.0001 p < 0.0001 White vs. Non-White p = 0.11 p = 0.02 ART Naive vs. ART Experienced p = 0.66 p = 0.26 Introduction of NNRTI (Yes vs. No) p = 0.57 p = 0.001 Concurrent PI Use (Yes vs. No) p = 0.91 p = 0.009 Clinically suspected HSR was influenced by several covariates Refined phenotype (patch test confirmed HSR) was independent of these covariates The SHAPE Study: Are Findings Relevant only to Caucasians? M Saag, et al. Clinical Infectious Diseases 2008; 46:1111-1118. Slide 22 Slide 23 SHAPE Study Key Results: Frequency of HLA-B*5701 by Patient Subgroups Percentage of Subjects with HLA-B*5701 (95% CI) For clinically suspected HSR, the frequency of HLA-B*5701 was lower in Black subjects For immunologically confirmed HSR, all case individuals carried the HLAB*5701 allele, regardless of race The utility of HLAB*5701 appears to generalize to US Black patients 100 100% 100% 80 60 40 44% 20 14% 4% White Black CS-HSR CS-HSR n=130 n=69 Clinically Suspected Cases White IC-HSR n=42 Black IC-HSR n=5 Immunologically Confirmed Cases White Control n=202 1% Black Control n=206 Abacavir Tolerant Controls (Adapted from: M Saag, et al. Clinical Infectious Diseases 2008; 46:1111-1118.) Develop / Access an Assay for Pharmacogenetic Marker Detection In general, development of laboratory based assays or test kits may be needed For HLA-B*5701: HLA typing was widely available for tissue typing purposes Several centralized clinical laboratories now offer HLA-B*5701 evaluation for abacavir hypersensitivity Lai-Goldman & Faruki, Genetics in Medicine 2008; 10: 874-78. Slide 24 Slide 25 Evaluate Cost Effectiveness A Cost Effectiveness Analysis of HLA-B*5701 Screening – Results depend on realistic assumptions – Usually, achieving a healthcare benefit comes with a cost – However, under many scenarios, screening with HLA-B*5701 was predicted to reduce HSRs and average cost of care (referred to as a “Dominant” outcome) Hughes, DA, et al., Pharmacogenetics 2004, 14:1–8 Slide 26 Educate & Inform Patients, Physicians, Regulators, and Payers Benefits & limitations of using the pharmacogenetic marker How to order the marker assay How to interpret the assay results For HLA-B*5701 key messages are: – Discontinue abacavir permanently if HSR cannot be ruled out, regardless of the HLA-B*5701 result. – HLA-B*5701 testing should never be performed diagnostically to support a decision to rechallenge with abacavir. – HLA-B*5701 testing must not be used as a screening test after someone has started treatment with abacavir. If a hypersensitivity reaction is suspected abacavir must be immediately and permanently discontinued. Slide 27 Challenges for Safety Pharmacogenetics Association of HLA-B*5701 with abacavir hypersensitivity effectively illustrates the potential of pharmacogenetics to improve drug safety, however… There are many challenges for safety pharmacogenetics: – Limited sample sizes in early clinical development – Very low rates of some serious adverse events – Monitoring safety of newly approved drugs – Acquiring DNA samples from cases – Accurate diagnosis of adverse drug reactions in the presence of symptoms from other causes – Finding causal markers that occur with low frequency – Finding multiple genetic markers contributing to risk of an adverse drug reaction Slide 28 Questions and Discussion