Polar Bond
advertisement
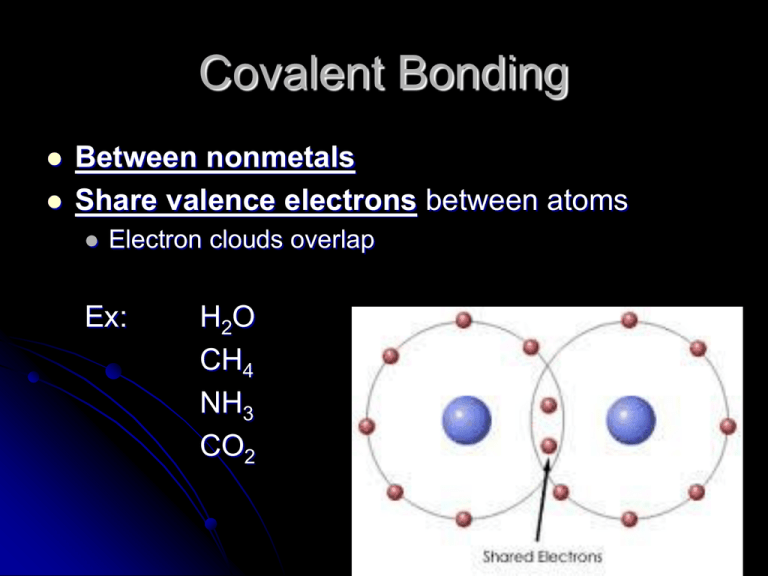
Covalent Bonding Between nonmetals Share valence electrons between atoms Electron clouds overlap Ex: H2O CH4 NH3 CO2 Electronegativity Difference in EN smaller than ionics and is usually < 1.7 Ex: HCl H = 2.2 Cl = 3.2 Difference = 1.0 Bond Polarity Polar Bond: there is a difference in EN values (unequal sharing) Ex: H EN=2.2 Cl EN=3.2 NonPolar Bond: no difference in EN values. (equal sharing) Ex: O2, N2, Cl2, H2 (all the diatomics!) Comparing Bond Types Single, Double, Triple Bonds Atoms can share single double or triple bonds between them. Each bond represents a shared pair of electrons. http://youtu.be/1wpDicW_MQQ Molecular Formulas Covalent compounds are molecules. Made up of all nonmetals. Molecular formulas: show actual number of atoms of each element present in compound Ex: H 2O 2 hydrogen atoms and 1 oxygen Structural Formulas Show how the atoms are bonded together in a covalent molecule. Use “lines” to show covalent bonds Empirical Formulas Show simplest whole number ratio of atoms or ions in the compound. Ex: MgCl2 1 : 2 ion ratio Ionic compounds are “salts” or ionic crystals. All ionic compounds have empirical formulas You can simplify some molecular formulas to make them empirical ratios Ex: C6H12O6 Simplest ratio of atoms CH2O Naming Covalent Compounds Prefix system indicates number of atoms of each element Mono Di Tri Tetra Penta Hexa Hepta Octa Nona Deca Molecule vs. Ionic Crystal CH4 = 5 atoms in molecule Formula Units vs. Molecules 7 minutes https://www.youtube.com/watch?v=dHWqJeSs8ms NaCl = 1:1 ion ratio Types of Chemical Formulas Tutorial https://www.youtube.com/watch?v=Y2eHJrZI t0k Naming Tutorial (Ionic & Covalent) https://www.youtube.com/watch?v=9XUsOLa z3zY Writing Formulas Tutorial (Ionic & Covalent) https://www.youtube.com/watch?v=16agvZ8 K2eM Drawing Covalent Molecules Lewis Structures See page 10 in Bonding Packet 1. 2. 3. 4. Count total valence e- in the molecule Draw molecule with single bonds between atoms (then subtract these e- from total) Evenly distribute remaining e- in pairs to all atoms in molecule that still need eCheck to see if all obey octet rule (Remember Hydrogen is fine with 2 e- ) 5. If deficient, shift over free e- pairs to make double or triple bonds as needed. Draw NH3 Draw H2O Draw CH4 Crash Course Chemistry: Lewis Structures: (11 minutes) http://www.youtube.com/watch ?v=a8LF7JEb0IA Drawing Polyatomic Ions Covalently bonded atoms with a group charge Follow same steps for drawing covalents. Add or subtract electrons from total valence depending on charge. Draw brackets around ion and indicate charge. Ex: (SO4) -2 6 + 4(6) + 2 = 32 electrons VSEPR and Molecular Shape To predict molecular shape, assume valence electrons repel each other. Molecule adopts 3D geometry that minimizes this repulsion. Valence Shell Electron Pair Repulsion (VSEPR) theory. Predicting Molecular Geometries Draw Lewis structure Count total number of electron pairs around the central atom (both shared and unshared) Arrange electron pairs to minimize e-repulsion Multiple bounds count as one bonding pair Molecular Shapes Regents Shapes to Know: Tetrahedral Pyramidal Bent Linear Is a Molecule Polar? If the centers of negative and positive charge do not coincide, then the molecule is polar. Look for Symmetry Polar Molecules: Have polar bonds and are not symmetrical Positive & negative “partial charges” don’t overlap They have a “dipole moment” Nonpolar Molecules Have nonpolar bonds OR Have polar bonds and are symmetrical Centers of positive & negative charge overlap Example: In CO2, the polarity of each C-O bond is cancelled because the molecule is linear. In H2O, the polar H-O bonds do not cancel because the molecule is bent. Tetrahedral Has 4 atoms bonded (no free pairs) Symmetry? Depends on what atoms are attached. Can be polar (asymmetrical) or nonpolar (symmetrical) Pyramidal Three atoms bonded (one free pair) Symmetry? All pyramids are asymmetrical. These molecules are always POLAR! Bent Two atoms attached (2 free pair) The 2 free pair make it bent and not linear. These are always asymmetrical so are always polar. H2O Hey, Water is Polar!!!!! Never forget this!!! Without polar water NO LIFE!! The polar nature of water molecules allows them to bond to each other in groups and is associated with the high surface tension of water. The polar nature of the water molecule has many implications. It causes water vapor at sufficient vapor pressure to depart from the ideal gas law because of dipole-dipole attractions. This can lead to condensation and phenomena like cloud formation, fog, the dewpoint, etc. It also has a great deal to do with the function of water as the solvent of life in biological systems Linear 2 atoms in molecule Ex: Cl Cl (nonpolar bond, sym., nonpolar molecule) H Br (polar bond, asym., polar molecule) 3 atoms in molecule Ex: CO2 GOOD FOR REVIEW Crash Course Chemistry: Polarity of Molecules http://www.youtube.com/watch?v=PVL24HAesnc Dipole Moment: https://www.khanacademy.org/science/organicchemistry/gen-chem-review/electronegativitypolarity/v/dipole-moment Properties of Covalent Compounds Melting Point Lower than Ionics To melt, you are only separating the weak bonds between molecules (not within). Melting Point Polar Molecules (dipoles/”mini-magnets”): Have higher melting points than non-polars because they are harder to separate. Solubility Polar Molecules dissolve in polar solvents as they are attracted to them like H2O, CHCl3, NH3 etc. Non-polar Molecules dissolve in non-polar solvents like hexane, CCl4 “Like Dissolves Like” Oil and water don’t mix! How does soap work? Conductivity Covalent Molecules do not conduct well as they do not form ions. They are “nonelectrolytes” Except Acids!!!! Acids are covalently bonded but in water (aqueous) they will ionize and conduct current. (Acids are not on this test) Decompose If the heat gets high enough covalent compounds will break down and decompose. (Remember the lab, sugar melted first, then it burned and turned into black carbon) Other Types of Covalent Bonds Coordinate Covalent Bonding Covalent bond in which one of the bonding atoms donates both of the electrons to the bond. The other atom donates nothing. Ex: Forming Hydronium Ion To form this type of bond you must have: A molecule with a free pair of electrons Something that needs to gain 2 electrons H+1 Ex: Forming Ammonium Ion Network Solids Giant network of covalently bonded atoms. Large macromolecules Extremely strong structures Unusually high M.P. Do not dissolve Diamonds are a giant network of carbon atoms. Ex: C (s) (graphite, diamond, buckyball), SiO2 (quartz), GeO2 “Buckyball” Bonding in Pure Metals Metallic Bonding Happens in pure metals or alloys. Ex: Mg, Fe, Brass, Au, Ni, Cu “Delocalized” valence electrons move about between all the metal atoms. http://www.youtube.c om/watch?v=XHV9L zCH2KA Metallic Bonding Properties Conducts heat and electricity very well Conducts as a solid too! Does not dissolve in solvents Malleable and Ductile Properties 2:00: http://www.youtube.co m/watch?v=srxNJ03W_ qM Relatively high melting point. Higher MP than covalents. Similar MP to most ionics What is a metal? 4:30 https://www.youtube.co m/watch?v=Xto88gMm Dzw Metallic Bonding http://www.drkstreet.com/resources/metallic-bondinganimation.swf EXTRA VIDEO LINKS FOR FUN AND LEARNING Ionic vs. Covalent Bonding http://youtu.be/QqjcCvzWwww http://youtu.be/yjge1WdCFPs Electronegativity http://youtu.be/Kj3o0XvhVqQ Bonding Dance Party http://www.youtube.com/watch?v=w BCmt_pJTRA It’s a chemical bond baby” http://youtu.be/wWUYHHo-zB0 Dancin Queen (Ionic/Covalent Bonds) http://youtu.be/BCYrNU-7SfA Isn’t it Ionic http://youtu.be/rwRtfrgJL5E




