Chapter 9 – Molecular Geometry
advertisement

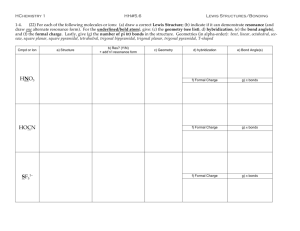
Chapter 9 Molecular Geometry Molecular Shapes Lewis Structures do not account for shape of molecules rather they only show the number and types of bonds between atoms Molecular Shapes The overall shape is determined by the bond angle, the angle made by the lines joining the nuclei of the atoms in a molecule Molecular Shapes We will focus on atoms with ABn format, a central atom A has n B atoms bonded to it Molecular Shapes VSEPR Model An electron domain consists of nonbonding pairs, single bonds, or multiple bonds VSEPR Model VSEPR Model Electron domains are negatively charged, so they repel each other, and stay out of each other’s way VSEPR Model the best arrangement of a given number of electron domains is the one that minimizes the repulsions among them VSEPR Model The shape of the substance in the ABn format depends on the number of electron domains surrounding the central atom (Electrondomain geometry) VSEPR Model Molecular shape describes the arrangement of atoms, not the electron domains (molecular geometry) VSEPR Model To Predict Shapes: 1. Draw Lewis structure and count e-d 2. Determine e-d geometry 3. Predict molecular geometry VSEPR Model See table VSEPR Model Domain s Basic Geometry 0 lone pairs 1 lone pair 2 lone pairs 3 lone pairs 2 Linear 3 trigonal planar bent tetrahedral trigonal pyramidal bent trigonal bipyramidal seesaw T-shaped octahedral square pyramidal square planar 4 5 6 linear VSEPR Model Sample Ex. 8.1-8.2 Use VSEPR to predict shape for the following substances: O3 SnCl3SeCl2 CO32IF5 ClF3 ICl4- VSEPR Model video VSEPR Model Nonbonding Electrons and Multiple Bonds can affect bond angles VSEPR Model E-ds for nonbonding electron pairs exert greater repulsive forces on adjacent e-ds and thus tend to compress bond angles VSEPR Model VSEPR Model Electron domains for multiple bonds exert a greater repulsive force on adjacent electron domains than single bonds VSEPR Model VSEPR Model VSEPR can be applied to larger molecules as well The shape can be predicted around individual atoms VSEPR Model VSEPR Model VSEPR Model Sample Exercise 9.3 Predicting Bond Angles Predict the approximate values for the H—O—C and O—C—C bond angles in vinyl alcohol Bond Polarity a measure of how equally electrons in a bond are shared based on electronegativity Dipole moment a measure of the charge separation in a molecule Dipole moment For a molecule with more than 2 atoms, the dipole moment depends on both polarities of the bonds and the geometry of the molecule. Dipole moment For ABn molecules in which all the B atoms are the same, certain symmetrical shapes will lead to nonpolar molecules even though the individual bonds are polar Dipole moment Nonpolar symmetrical shapes are linear, trigonal planar, tetrahedral, square planar, trigonal bipyramidal, and octahedral Dipole moment Dipole moment Dipole moment Sample Exercise 9.4 Polarity of Molecules Predict whether the following molecules are polar or nonpolar: (a) BrCl (b) SO2 (c) SF6 (d) NF3 (e) BCl3 Hybrid orbitals Hybridization is a process that occurs when atomic orbitals mix to form new orbitals called hybrid orbitals and form bonds Hybrid orbitals Hybrid orbitals Predicting hybridization 1. Draw lewis structure 2. Determine # e-d 3. Specify the hybrid orbitals needed to accommodate the electron pairs based on their geometric arrangement Predicting hybridization # e-d 2 3 4 5 6 hybridization sp sp2 sp3 dsp3 d2sp3 Sample Exercise 9.5 Hybridization Indicate the hybridization of orbitals employed by the central atom in (a) NH2– (b) SF4 (c) SO32– (d) SF6 MULTIPLE BONDS All of the single bonds we have looked at are called sigma (s) bonds, where there is an overlap in the middle of the region of s and p orbitals. Multiple Bonds To describe multiple bonds, we must look at the side to side overlapping of 2 p orbitals, called a pi (p) bond Multiple Bonds double bond is one s bond and one p bond A Multiple Bonds triple bond is one s bond and two p bonds A Multiple Bonds Multiple Bonds When looking at molecules that exhibit resonance there are delocalized p bonding Sample Exercise 9.6 Describing s and p Bonds in a Molecule Formaldehyde also known as ? has the following Lewis structure . Count the s and p bonds. Tell me everything you can about Acetonitrile Review PF5 Review CO2 Review ICl4 - Review AsH3 Review I3 - Review SO2 Review IF5 Review CO3 2- Review SF6 Review ClO4 Review OF2 Review IF4 + Review ClF3 Review NCS Review Directions: Predict the molecular geometry, bond angle, hybridization, s and p bonds, and polar/nonpolar for the following molecule : Methanoic acid Methyl Amine Ethyne