CH 2 - nchsdduncanchem2
advertisement

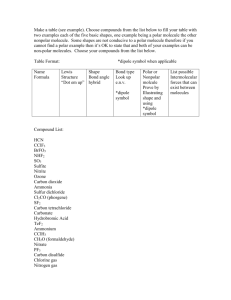
Bonding and Percent Name: _____________________________________ Page 1 of 5 WS E: Lewis Structure Worksheet: See me to see which ones you have to do. Draw the Lewis Structures You may need to watch the bonding videos Determine the shape in the Chem1 folder on the desk top. Determine the polarity of the molecule 1. HCl 9. OI2 2. Br2 10. CS2 3. SeBr2 11. SiBr4 4. CF4 12. F2 5. PI3 13. HCN 6. O2 14. NH4 + 7. N2 15. NO2 – 8. H2 16. SO3 Bonding and Percent 17. SO4 2- Name: _____________________________________ Page 2 of 5 26. OCN- 18. NO3- 27. SCN- 19. PO33- 28. O3 20. CN- For the following structures: You do NOT have to determine the shapes 29. H3CCOOH 21. CO2 30.CH3CH2OH 22. CO 31.H3COCH3 23. I2 32.H3CCH3 24. CO32- 33.H2CCH2 25. SO2 34.HCCH Bonding and Percent 35. C2H4 Name: _____________________________________ Page 3 of 5 43. CH3NH2 (Explanation: 1st C has 3 H’s and one N. The N has 2 H’s.) 36. CH2CCH2 (Explanation for CH2CCH2: The notation here has organization to it. It is written this way because the 1st C has 2 H’s attached the second C as NO H’s attached and the third C has 2 H’s attached.) 44. (CH2)5 45. As 37. HCN 38. CH3COCH3 39. Cl2 40. P2 41. PCl3 42. CCl3F Bonding and Percent WS F: Intermolecular Forces Substance #1 Name: _____________________________________ Page 4 of 5 Predominant Intermolecular Force Substance #2 (a) HCl(g) I2 (b) CH3F CH3OH (c) H2O H2S (d) SiO2 SO2 (e) Fe Kr (f) CH3OH CuO (g) NH3 CH4 (h) HCl(g) NaCl (i) SiC Predominant Intermolecular Force Cu 2. Rank the following substances in order from lowest to highest melting point. CO2, NaCl, Ag, H2O, He, HBr 3. Rank the following substances in order from lowest to highest freezing point. H2O, Ca3(PO4)2, Cr, C2H6, OF2 4. Rank the following substances in order from highest to lowest boiling point. Cl2, Ne, Ca, Cr(OH)3, CH3CH2OH, Diamond Substance with Higher Boiling Point Bonding and Percent Name: _____________________________________ Page 5 of 5 Molecular Shapes “clouds” 2 Linear Diatomic Polarity depends upon electronegativity difference Polar if >0.5 Nonpolar if <0.5 Linear Triatomic, Usually nonpolar CO2, HCN 3 Trigonal Planar: BF3, SO3, NO3120˚ Usually nonpolar In molecules where the outside molecules are different, shapes that tend to be nonpolar usually become polar. Remember to count the number of “clouds” of electrons, not the actual number of electrons. A double or triple bond counts as one effective pair. Bent, 12O˚ Usually polar NO2- Also: If there ever is a two molecule atom (diatomic) that molecule’s polarity depends upon the electronegativity difference of the atoms 4 Tetrahedral; 109˚: Usually nonpolar CH4, CF4 Pyrimidal: 107˚ Usually polar: NH3, PCl3 Bent: 104.5˚ Usually polar: H2O, OF2 Bond Ionic (85) Covalent Polar Molecules Nonpolar Molecules H-Bond (5) Dipole Force (1) LDF (0.1) Metallic (65) Network Solids (100)