Molecular Shapes
advertisement

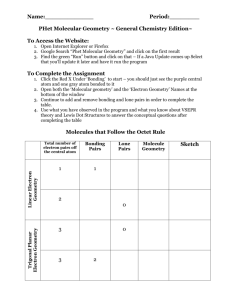
Drawing Lewis structures 1. Calculate the total number of valence electrons in the compound. 2. Choose the central atom and place the remaining atoms symmetrically around the central atom. 3. Place one electron pair (or a line) between each pair of bonded atoms. 4. Complete the octet of each atom (duplet for H) by placing the remaining valence electron as electron pairs around the atoms. 5. If there are not enough electrons form multiple bonds. Shapes of Molecules • Electron pairs surrounding an atom repel each other. This is referred to as Valence Shell Electron Pair Repulsion (VSEPR) theory. • The electron pair geometry indicates the arrangement of bonding and nonbonding electron pairs around the central atom. • The molecular shape gives the arrangement of atoms around the central atom as a result of electron repulsion. How To Use VSEPR Model? 1. Draw the Lewis structure 2. Identify the central atom and assign the letter A to the central atom. 3. Count the total number of electron pairs (bonding and nonbonding) around the central atom. Each lone pair will be assigned the letter E, and each bonding pair will be assigned the letter X. Treat a multiple bond equivalent of a single electron pair 3. Arrange the electron pairs in a way to minimize e--e- repulsion. The lone pairs must be as far as possible from one another and from bond pairs. Shape of Molecules AXnEm A is the central atom n is the number of bonding pairs, m the number of non bonding pairs n m Example Shape 2 0 BeH2 Linear 3 0 BH3 Trigonal Planar 4 0 CH4 Tetrahedradral 2 1 HNO Bent 3 1 NH3 Trigonal Pyramidal 2 2 H2O Bent Linear Molecule Bent Molecule Tetrahedral Molecule Trigonal Pyramidal Molecule Tetrahedral Molecules • Methane, CH4, has four pairs of bonding electrons around the central carbon atom. • The four bonding pairs (and therefore atoms) are repelled to the four corners of a tetrahedron. The electron pair geometry is tetrahedral. • The molecular shape is also tetrahedral. Trigonal Pyramidal Molecules • In ammonia, NH3, the central nitrogen atom is surrounded by three bonding pairs and one nonbonding pair. • The electron pair geometry is tetrahedral and the molecular shape is trigonal pyramidal. Bent Molecules • In water, H2O, the central O atom is surrounded by two nonbonding pairs and two bonding pairs. • The electron pair geometry is tetrahedral and the molecular shape is bent. Linear Molecules • In carbon dioxide, CO2, the central C atom is bonded to each oxygen by two electron pairs (a double bond). • According to VSEPR, the electron pairs will repel each other, and they will be at opposite sides of the C atom. • The electron pair geometry and the molecular shape are both linear. Summary of VSEPR Theory Molecular Polarity • In water, the molecule is not linear and the bond dipoles do not cancel each other. • Therefore, water is a polar molecule. Polar Bond The overall polarity of a molecule depends on its molecular geometry Conclusions Continued • VSEPR Theory can be used to predict the shapes of molecules. • The electron pair geometry gives the arrangement of bonding and nonbonding pairs around a central atom. • The molecular shape gives the arrangement of atoms in a molecule. • The molecular shape gives information about the polarity of the molecule.