Atomic Theory Models of the Atom
advertisement

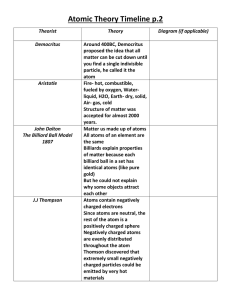
Quiz Time! You have 5 minutes to go over the following: Parts of an atom Calculating protons/neutrons/electro ns Drawing Bohr models! Austin High School Chemistry 2015-2016 The idea that all matter is made up of atoms. It is a very old idea dating back to the ancient Greeks. Over time, scientists have come up with various models for the atom based on their observations. These atomic models have been altered and revise as new scientific evidence is discovered. Democritus was an ancient Greek philosopher who lived from 460 370 B.C. He proposed: Matter is formed of small pieces that could not be cut into smaller parts called “ATOMS” meaning uncut-table. Atoms are small hard particles. Made of a single material that’s formed into different shapes and sizes. • 2500 years later a British chemist and schoolteacher brings back Democritus’s idea of the atom • He performed many experiments to study how elements join together to form new substances Atoms are tiny invisible particles. Atoms of one element are all the same. Atoms of different elements are different. 4. Compounds form by combing atoms. 1. 2. 3. • Discovered negatively charged particles with the cathode ray tube (electrons). • Measured the charge to mass ratio of the electron •Knew there had to be other particles in atoms (because of the mass). Passing an electric current through the cathode makes a beam appear to move from the negative to the positive end. Electrons are negatively charged and are attracted to a positive magnetic source. Atoms are made mostly out of (+) charged materials, like dough in a bun. The (-) charged electrons are found inside the (+) dough. 10 Rutherford's 'gold foil' experiment using positively charged alpha particles: Most alpha particles passed through the gold foil suggesting that an atom is largely empty space. Some alpha particles were deflected significantly suggesting that the positive charge of an atom must be concentrated in a very small sphere. A very small number of alpha particles actually bounced back. Alpha particles are deflected if they get close enough to the nucleus 11 Proposed that electrons move around the nucleus in specific layers or shells. Every atom has a specific number of electrons shell. An electron can absorb energy and move to a higher energy orbit of larger radius. (excited electrons) An excited electron can fall back to its original orbit by emitting energy as radiation. 13 Chadwick discovered the neutron which is a particle with no charge, found in the nucleus. 15