Measurement Scramble PowerPoint
advertisement
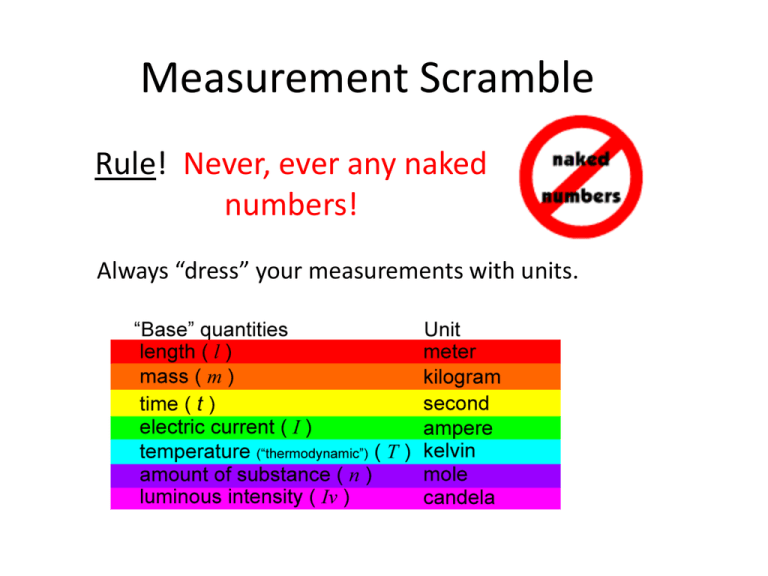
Measurement Scramble Rule! Never, ever any naked numbers! Always “dress” your measurements with units. 8 Stations Some are at your desk and others are at stations: 1. 2. 3. 4. 5. 6. 7. 8. Length & Distance Time Mass Weight Temperature Solid Volume Liquid Volumes Density 1. Length & Distance (At Station #1) • • • • Length = the amount of vertical space an object takes up Distance = the degree or amount of separation between two points, lines, surfaces, or objects Base unit = meters (m) Inquiry Question: What is the effect of mass on kinetic energy as measured by the distance the cart can push the wood block? Hypothesis: If weights are added to a cart, then the kinetic energy will be ___________ and the block of wood will be ________________________. Cart Mass (g) Distance Block moved (cm) Without weight With weights Ramp Wood Block 2. Time – (At Desk) • • • Time = a dimension in which events can be ordered from the past through the present into the future, and also the measure of durations of events and the intervals between them Base Unit = seconds (s) Inquiry Question: What is the effect of gender on average reaction time of Middle School students’ hands as measured by the amount of time needed to catch a dropped meter stick? http://www.youtube.com/watch?v=CYHrPvDnGTY Hypothesis: If a meter stick is dropped between a ________________ Middle School students’ thumb and forefinger, the reaction rate will be ____________________________________. Student Trial 1 (s) Trial 2 (s) Average (s) 3. Mass – (At Station # 3) • Mass = the amount of matter • Base Unit = grams (g) • Task: Measure the mass of a material without removing the material from the container. 4. Weight – (At Station #4) • Weight = the force on the object due to gravity; mass x gravitational acceleration; w = m x g • On Earth: gravitational acceleration = g = .098 cm/s2 • Base Unit = Newtons (N) • Task: Compare the measured weight of a toy car with the calculated weight of a toy car. Toy car mass = _______ g 5. Temperature – (Take Tray to Desk) • Temperature = Temperature is a measure of the internal energy of the system, while heat is a measure of how energy is transferred from one system (or body) to another. • Base Unit = degrees Celsius (° C) • Task: Measure the temperature change in the following endothermic chemical reaction. H3C6H5O7+ 3 NaHCO3→ 3 CO2+ 3 H2O+ Na3C6H5O7 Citric acid 100 ml Baking Soda 1 level teaspoon 6. Solid Volume – (At Desk) • • • • Volume = the amount of space an object takes up Base unit = cubic meter (m3) Important Conversion: 1 ml = 1 cm3 Task: Calculate the volumes for 2 materials: Regular Shape Irregular shape 7. Liquid Volume – (At Desk) • Liquid Volume = space a substance takes up in a container • Base Unit = liter (L) • Task: Practice measuring out the following amounts of water using the different graduated cylinders. 2 ml 25 ml 50 ml Remember! : Your eye should be level With the meniscus. 8. Density – (Station # 8 Triple Beam Balance) • Density = the amount of mass per unit of volume; d = mv • Base unit = gram per cubic centimeter g/cm3 • Density of water (@ room temperature) = 1 g/cm3 • Inquiry Question: What effect does density have on whether or not a material will float in water? Hypothesis: If a material is _______ dense than water, then it ___________________ float in water. Directions: 1) Use Triple Beam to measure mass of each cube in grams. 2) Use protractor to measure side of cube in cm. 3) Return to desk to do calculations. Density = mass/ volume