Methane
advertisement

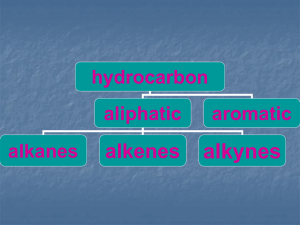
Methane Hydrocarbons – compounds containing only carbon and hydrogen. hydrocarbons aliphatic alkanes alkenes aromatic alkynes Alkanes – hydrocarbons with the general formula CnH2n+2 (four bonds to each carbon and only single bonds) CH4 methane C2H6 ethane C3H8 propane Etc. Methane = CH4 H | H—C—H | H sp3 tetrahedral 109.5o bond angles Non-polar – van der Waals (London forces) Gas at room temperature mp = -183oC bp = -161.5oC Water insoluble Colorless and odorless gas “swamp gas” ; fossil fuel found with petroleum & coal Important fuel/organic raw material Chemistry of methane (reactions)? CH4 + H2O NR (no reaction) CH4 + conc. H2SO4 NR CH4 + conc. NaOH NR CH4 + sodium metal NR CH4 + KMnO4 NR CH4 + H2/Ni NR CH4 + Cl2 NR Methane is typically unreactive. It does not react with water, acids, bases, active metals, oxidizing agents, reducing agents, or halogens. Reactions of methane: 1. Combustion (oxidation;complete & partial) 2. Halogenation Reactions of Methane 1. Combustion (oxidation) a) complete oxidation CH4 + 2 O2 , flame or spark CO2 + H2O + energy b) partial oxidation 6 CH4 + O2 , 1500o CH4 + H2O , Ni, 850o CO + H2 + H2C2 (acetylene) CO + H2 2. Halogenation CH4 + X2 , Δ or hυ CH3X + HX X2 = Cl2 or Br2 a) Requires heat (Δ) or uv light (hυ) b) May proceed further c) Cl2 reacts faster than Br2 d) No reaction with I2 “Substitution” reaction NR CH4 + Cl2 CH4 + I2, heat NR CH4 + Br2, hv (requires heat or uv light) (does not react with I2) CH3Br + HBr CH4 + Cl2, hv CH3Cl + HCl methyl chloride chloromethane CH3Cl + Cl2, hv CH2Cl2 + HCl methylene chloride dichloromethane CH2Cl2 + Cl2, hv CCl3H + HCl chloroform trichloromethane CCl3H + Cl2, hv CCl4 + HCl carbon tetrachloride tetrachloromethane CH4 + Br2, hv CH3Br + HBr methyl bromide bromomethane CH3Br + Br2, hv CH2Br2 + HBr methylene bromide dibromomethane CH2Br2 + Br2, hv CBr3H + HBr bromoform tribromomethane CBr3H + Br2, hv CBr4 + HBr carbon tetrabromide tetrabromomethane CH3I CH2I2 iodomethane diiodomethane methyl iodide methylene iodide CHI3 triiodomethane iodoform CI4 tetraiodomethane carbon tetraiodide Can proceed further: CH4 + Cl2, heat CH3Cl + CH2Cl2 + CHCl3 Control? (xs) CH4 bp –162o CH4 + Cl2, heat CH3Cl + HCl bp –24o + (xs) Cl2, heat CCl4 + 4 HCl + CCl4 + HCl Mechanism step 1 : initiating step = homolytic bond dissociation Cl Cl step 2 H + H C H H . Cl Cl Cl . + Cl Cl . + . Cl Cl H Cl Cl . + + H C H H . Cl possible but non-productive step 3 H H C H . + Cl Cl H H C Cl H + .Cl Mechanism for the monochlorination of methane initiating step: 1) Cl2 2 Cl• propagating steps: 2) Cl• + CH4 HCl + CH3• 3) CH3• + Cl2 CH3Cl + Cl• then 2), then 3), then 2), etc. terminating steps: 4) Cl• + Cl• Cl2 5) Cl• + CH3• CH3Cl 6) CH3• + CH3• CH3CH3 Energy Changes? ΔH Homolytic bond dissociation energies (see inside the front cover of M&B) H—Cl 103 Kcal/mole Cl—Cl 58 Kcal/mole CH3—H 104 Kcal/mole CH3—Cl 84 Kcal/mole We need only consider those bonds that are broken or formed in the reaction. CH3—H + Cl—Cl CH3—Cl + H—Cl +104 +58 -84 -103 PE: +162 -187 ΔH = +162 –187 = -25 Kcal/mole (exothermic, gives off heat energy) ΔH for each step in the mechanism? 1) Cl—Cl 2 Cl• +58 ΔH = +58 2) Cl• + CH3—H H—Cl + CH3• +104 -103 ΔH = +1 3) CH3• + Cl—Cl CH3—Cl + Cl• +58 -84 ΔH = -26 4) Cl• + Cl• Cl—Cl -58 ΔH = -58 Rates of chemical reactions depend on three factors: Collision frequency (collision per unit time) Probability factor (fraction of collisions with correct geometry) Energy factor (fraction of collisions with sufficient energy) “sufficient energy” = Energy of activation, minimum energy required for a collision to go to the product. rate Z * P * e Eact/RT Z = collision frequency P = probability factor e-Eact/RT = fraction of collisions with E > Eact Note: rate decreases exponentially as the Eact increases! @ 275oC Eact Collisions > Eact 5 Kcal 10,000/1,000,000 10 Kcal 100/1,000,000 15 Kcal 1/1,000,000 If the Eact is doubled, the rate is decreased by a factor of 100 times! Eact cannot be easily calculated like ΔH, but we can estimate a minimum value for Eact: If ΔH > 0, then Eact > ΔH If ΔH < 0, then Eact > 0 Rate determining step (RDS) = the step in the mechanism that determines the overall rate of a reaction. In a “chain reaction” this will be the slowest propagating step. For chlorination of methane, which propagating step is slower? Step 2) ΔH = +1 Kcal/mole Eact > +1 Kcal (estimated) Step 3) ΔH = -26 Kcal/mole Eact > 0 Kcal (estimated) Step 2 is estimated to be slower than step 3 and is the RDS An “alternate mechanism: 2) Cl• + CH4 CH3Cl + H• 3) H• + Cl2 HCl + Cl• Why not this mechanism? Step 2: ΔH = +104-84 = +20 Kcal/mole; Eact > +20 Kcal Step 3: ΔH = +58-103 = -45 Kcal/mole; Eact > 0 Kcal RDS for this mechanism is step 2 and requires a minimum of 20Kcal/mole! Unlikely compared to our mechanism where the RDS only requires an estimated minimum of 1 Kcal! 2. Halogenation Δ or hυ CH4 + X2 CH3X + HX requires heat or light X2: Cl2 > Br2 I2 why?…how?…mechanism This reaction requires heat or light because the first step in the mechanism involves the breaking of the X-X bond. This bond has to be broken to initiate the chain mechanism. F—F 38 Kcal/mole Cl—Cl 58 Kcal/mole Br—Br 46 Kcal/mole I—I 36 Kcal/mole Once initiated the reaction may or may not continue based on the Eact for the RDS. “generic” mechanism for the halogenation of methane (free radical substitution mechanism) 1) X2 2 X• 2) X • + CH4 3) CH3• + X2 CH3X HX 4) 2 X• X2 5) X• + CH3• CH3X 6) 2 CH3• CH3CH3 + CH3• + X• ΔH for each step in the mechanism by halogen: F Cl Br I 1 +38 +58 +46 +36 2 -32 +1 +16 +33 3 -70 -26 -24 -20 4 -38 -58 -46 -36 5 -108 -84 -70 -56 6 -88 -88 -88 -88 Estimation of Eact for the propagating steps: Eact (est.) F Cl Br I 2 >0 >+1 >+16 >+33 3 >0 >0 >0 >0 Step 2 is the RDS Rate Cl2 > Br2 because in the RDS Eact(Cl2) < Eact(Br2) NR with I2 because RDS Eact(I2) > +33 Kcal/mole only 1/1012 collisions would have E > +33 at 275o The transition state (‡) or “activated complex” is the unstable structure that is formed between reactants and products in a step in a mechanism. It corresponds to the energy at the top of the energy barrier between reactants and products. step 2 in the chlorination of methane: Cl• + CH4 HCl + CH3• Transition state: [ Cl--------H-------CH3 ]‡ δ• δ• Hammond’s Postulate: the higher the Eact of a step in a mechanism, the later the transition state is reached and the more the transition state will look like the products. In step 2 of the mechanism for the bromination of methane, the Eact is estimated to be > +16 Kcal/mole. Since the Eact is high, the transition state is reached later in this step than it is in chlorination and will look more like the products: [ Br----H-----------CH3 ]‡ δ• δ• Reactions of Methane 1. Combustion (oxidation) a) complete oxidation CH4 + 2 O2 , flame or spark CO2 + H2O + heat b) partial oxidation 6 CH4 + O2, 1500oC CO CH4 + H2O, 850o, Ni + H2 CO + + H2C2 H2 2. Halogenation CH4 + X2, heat or hv requires heat or light Cl2 > Br2 NR with I2 CH3X + HX