chapter 3 - Doral Academy Preparatory
advertisement

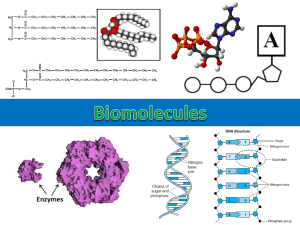
CHAPTER 3 BIOCHEMISTRY CARBON COMPOUNDS • Although water is the primary medium for life on earth, most of the molecules from which living organisms are made are based on the element carbon. • All compounds can be classified in two broad categories: organic compounds and inorganic compounds • Organic compounds are made primarily of carbon atoms. • Inorganic compounds do not contains carbon atoms. CARBON BONDING • A carbon atom has 4 electrons in its outermost energy level, therefore readily forms four covalent bonds with the atoms of other elements. • Unlike other elements, however, carbon also bonds with other carbon atoms forming straight chains, branched chains, or rings. FUNCTIONAL GROUPS • In most organic compounds, clusters of atoms, called functional groups, influence the characteristics of the molecules they compose and the chemical reactions the molecules undergo. • The common functional groups: - Hydroxyl - Carboxyl - Amino - phosphate LARGE CARBON MOLECULES • Many carbon compounds are built up from smaller, simpler molecules called monomers. • A polymer is a molecule that consists of repeated, linked monomers. • Large polymers are called macromolecules. Condensation reaction • Monomers link to form polymers through a chemical reaction called a condensation reaction. • Each time a monomer is added to a polymer, a water molecule is released. hydrolysis • In addition to building polymers through condensation reactions, living organisms also have to break down polymers. • The breakdown of polymers occurs through a process called hydrolysis. MOLECULES OF LIFE • Four main classes of organic compounds are essential to the life processes of all living things: (macromolecules) 1. 2. 3. 4. Carbohydrates Proteins lipids Nucleic acids CARBOHYDRATES • Carbohydrate is a fancy way of saying "sugar.“Scientists came up with the name because the molecule has many carbon (C) atoms bonded to hydroxide (OH-) groups. • What's It Used For? A carbohydrate is called an organic compound, because it is made up of a long chain of carbon atoms. Sugars provide living things with energy and act as substances used for structure. • Saccharides Scientists also use the word saccharide to describe sugars. If there is only one sugar molecule, it is called a monosaccharide. If there are two, it is a disaccharide. If there are three, it is a trisaccharide. You get the idea. • Carbohydrates can exist as: 1. Monosaccharides – a monomer of a carbohydrate (simple sugar) - formula: (CH2O)n n= any whole number from 3 to 8. * The most common monosaccharide are glucose ,fructose and galactose 2. Disaccharide – two monosaccharides can combine in a condensation reaction to form a double sugar. Ex: the monosaccharides fructose and glucose can combine to form the dissaccharide sucrose. 3. Polysaccharide – a complex molecule composed of three or more monosaccharides. Ex: the polysaccharide glycogen, consists of hundreds of glucose molecules. PROTEINS Proteins are made of amino acids. Even though a protein can be very complex, it is basically a long chain of amino acid subunits all twisted around like a knot. • Amino Acids – There are 20 different amino acids. Amino acids are used in every cell of your body and are used to build the proteins you need to survive. • The side groups (R-group) are what make each amino acid different from the others. Amino acid structure • All amino acids share a basic structure. Each amino acid contains a central carbon atom, covalently bonded to 4 other atoms or functional groups. A hydrogen atom (H), an amine group (NH2 ), a carboxyl group (COOH) and a side chain/R –group. Dipeptides & Polypeptides • Dipeptide – In a condensation reaction, two amino acids bond to form a dipeptide. The two amino acids form a covalent bond called a peptide bond. • Polypeptide- very long chains of several amino acids. • Proteins are composed of one or more polypeptides. Enzymes • Enzyme-RNA or protein molecules that act as biological catalysts. (catalyst- reduce the amount of activation energy that is needed for a reaction to take place.) • Enzyme reactions depend on the fit between the substrate and the enzyme. The folds on the enzymes are the active site where the substrate binds. Enzyme structure LIPIDS • When you think of fats, you should know that they are lipids. Lipids are also used to make steroids and waxes. So, if you pick out some earwax and smell it, that's a lipid, too! • Lipids include: triglycerides, phospholipids, steroids and waxes. • Fatty acids long carbon chains that make up most lipids. The two ends of the fatty acid molecule have different properties. The carboxyl end is hydrophilic (attracted to water molecules). The Carbon end is hydrophobic (water fearing). • Triglycerides- composed of three molecules of fatty acid joined to one molecule of the alcohol glycerol. • Ex: butter, Fats in red meat • Phospholipids – composed of two fatty acids attached to a molecule of glycerol. Ex: cell membrane • Waxes- type of structural lipid consisting of a long fatty-acid chain joined to a long alcohol chain. Waxes are used to coat and protect things in nature. • Steroids- molecules composed of four fused carbon rings with various functional groups attached to them. NUCLEIC ACIDS • Nucleic acids are very large complex organic molecules that store and transfer important information in the cell. • Two major types: -Deoxyribonucleic acid (DNA) - Ribonucleic acid (RNA) DNA- contains information that determines the characteristics of an organism and directs cell activity. RNA – stores and transfers information from DNA that is essential for the manufacturing of proteins. • Both DNA and RNA are composed of thousands of linked monomers called nucleotides. Each nucleotide is made of a phosphate group, a five carbon sugar, and a ring-shaped nitrogenous base.