The 1st Law, Temperature Changes, Heat Capacity
advertisement

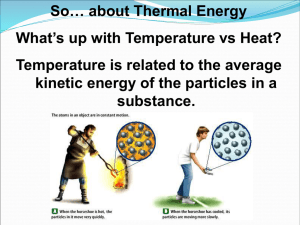
• • • • Last time… Course policies Thermodynamics Types of Energy First Law of Thermodynamics (Conservation of Energy) E 0 u n iverse Energy Units J 1J= kJ cal kcal BTU 0.2390 2.390 x 10-4 kWh 1 0.001 1 kJ = 1000 1 239.0 0.2390 1.0557 2.778 x 10-4 1 cal = 4.184 4.184 x 10-3 1 0.001 252 1.162 x 10-6 1 kcal = 4184 4.184 1000 1 0.252 1.162 x 10-3 1 kWh = 3.6 x 106 3.6 x 103 8.604 x 105 860.4 Read as 1J = 0.2390 cal 1055 2.778 x 10-7 2.93 x 10-4 1 Example-Energy Unit Conversion •The amount of energy required to pump one sodium ion out of a cell is 114 “milli electron Volts” •114meV=114 x 10-3eV •1eV = 1.602 x 10-19J The Chocolate Chip Challenge: How many grams of salt would you need to give you the number of sodium ions that could be transported by the energy in one chocolate chip? (1 chocolate chip has 3.5 Calories) Temperature and Heat Heat (q) and Temperature (T) are not the same! The more thermal energy something has, the greater the motion of its atoms The total thermal energy in an object is the sum of the individual energies of all the atoms, molecules, or ions Which statement below best describes the process of placing a thermometer initially at 22ºC into ice water? 1. Some of the thermal energy of the ice water is transferred to the thermometer. 2. Some of the thermal energy of the thermometer is transferred to the ice water, melting some of the ice. 3. The atoms of mercury begin to move faster as a result of the thermal energy transfer between the thermometer and the ice water. 4. The mercury in the thermometer begins to expand as a result of the thermal energy transferred. 43% 27% 16% 1 14% 2 3 4 What happens to thermal (heat) energy? Warms another object (transfer) Causes a change of state Is used in an endothermic reaction Heat Transfer If heat (q) is transferred, in which direction does it go? From hotter to cooler (related to the 2nd Law, but we’ll get to that later) Heat lost = Heat gained (1st Law) q q warmer co o l er Thermal equilibrium= when two objects in contact reach the same temperature System and Surroundings System = Thing or things being studied Isolated: Neither energy or matter can be transferred to surroundings Closed: Energy, but not matter can be transferred to surroundings Surroundings = Everything else in the universe Energy transfer between system and surroundings Internal energy=Energy of a closed system We can measure changes Change occurs if heat is transferred to/from system or if work is done on/by system Esystemqw qheat wwork How to Determine the Sign of q and w + Endothermic- system takes in heat Ammonium thiocyanate and barium hydroxide hydrate Exothermic- system gives off heat System E=q+w Heat transferred in q>0 Heat transferred out q<0 Work done on w>0 Work done by w<0 Endothermic Exothermic - Internal Energy Change Example: A gas is compressed and during this process the surroundings do 143 J of work on the gas. At the same time, the gas absorbs 212 J of heat from the surroundings. What is the change in the internal energy of the gas? Heat Transfer and Specific Heat Capacity When I heat an object, what happens and how much energy does it require? It depends . . . 1. Quantity (How much stuff do I have?) 2. Amount of heat energy added 3. Identity of the material Heat capacity is the energy needed to raise 1g by 1ºC. q m C T q n C T How much energy does it take to boil water for tea? The instructions for baking brownies say to heat the oven to 350ºF if using an aluminum pan, but to heat the oven to 325ºF if using a glass pan. Why is this? Glass heats up faster because it has a lower heat capacity. Glass heats more slowly because it has a lower heat capacity. Aluminum heats more slowly because it has a lower heat capacity. Aluminum heats up faster because it has a higher heat capacity. The instructions for baking brownies say to heat the oven to 350ºF if using an aluminum pan, but to heat the oven to 325ºF if using a glass pan. Why is this? 1. 2. 3. 4. Glass heats up faster because it has a lower heat capacity. Glass heats more slowly because it has a lower heat capacity. Aluminum heats more slowly because it has a lower heat capacity. Aluminum heats up faster because it has a higher heat capacity. 75% 16% 2% 1 2 3 7% 4 Enthalpy Quantitative: Calculating Heat Exchange: Specific Heat Capacity Temperature Changes from Heat Exchange Example 1: 5 g wood at 0 oC Example 2: 10 g wood at 0 oC Example 3: 5 g copper at 0 oC Example 4: 5 g wood at 0 oC Clicker Choices: 1: 0 oC 2: 33 oC 3: 50 oC + + + + 4. 67 oC 5 g wood at 100 oC 5 g wood at 100 oC 5 g copper at 100 oC 5 g copper at 100 oC 5: 100 oC 6: other What happens to thermal (heat) energy? When objects of different temperature meet: Warmer object cools Cooler object warms Thermal energy is transferred qwarmer = -qcooler specific heat x mass x T = specific heat x mass x T warmer object cooler object Heat transfer between substances: J o q = 1 . 8 5 g ( 1 8 C ) w o o d o g C = 1 6 0 J J o q = 0 . 3 8 5 5 g ( + 8 2 C ) C u o g C = + 1 6 0 J Example If we mix 250 g H2O at 95 oC with 50 g H2O at 5 oC, what will the final temperature be? Thermal Energy and Phase Changes First: What happens? Thermal Energy and Phase Changes First: What happens? Thermal Energy and Phase Changes First: What happens? But what’s really happening? Warming: • Molecules move more rapidly • Kinetic Energy increases • Temperature increases Melting/Boiling: • Molecules do NOT move more rapidly • Temperature remains constant • Intermolecular bonds are broken • Chemical potential energy (enthalpy) increases Energy and Phase Changes: Quantitative Treatment Melting: Heat of Fusion (Hfus) for Water: 333 J/g Boiling: Heat of Vaporization (Hvap) for Water: 2256 J/g Total Quantitative Analysis Convert 40.0 g of ice at –30 oC to steam at 125 oC Warm ice: (Specific heat = 2.06 J/g-oC) Melt ice: Warm water (s.h. = 4.18 J/g-oC) Total Quantitative Analysis Convert 40.0 g of ice at –30 oC to steam at 125 oC Boil water: Warm steam (s.h. = 1.92 J/g-oC) Enthalpy Change and Chemical Reactions H = energy needed to break bonds – energy released forming bonds Example: formation of water: H = ? Enthalpy Change and Chemical Reactions H is usually more complicated, due to solvent and solid interactions. So, we measure H experimentally. Calorimetry Run reaction in a way that the heat exchanged can be measured. Use a “calorimeter.” Calorimetry Experiment N2H4 + 3 O2 2 NO2 + 2 H2O Energy released = E absorbed by water + E absorbed by calorimeter Ewater = Ecalorimeter = Total E = H = energy/moles = 0.500 g N2H4 600 g water 420 J/oC Hess’s Law If reactions can be “added” so can their H values. Fig. 5-4, p. 183 Table 5-2, p. 195