Periodic Table and Periodic Trends
advertisement

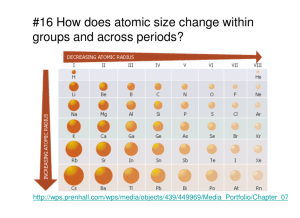
Turn to page 3 in the Unit Note Packet History of the Periodic Table In the mid-1800’s, Dimitri Mendeleev published a table of all of the known elements, 70 elements to be exact. Not until Dmitri Mendeleev, no one had come up with a way to organize the elements. Mendeleev came up with the first working system of filing the elements. Mendeleev’s table was arranged based on the increasing atomic mass. He listed the elements with similar chemical and physical properties in columns on his periodic table. They were in order of increasing atomic mass. History of the Periodic Table Mendeleev left gaps in the table since there were no current elements that seemed to fit those spots Those elements were eventually discovered and they fit perfectly into an open spot. Today, we list the elements in order of increasing atomic number. We owe this arrangement to Henry Moseley History of the Periodic Table The modern Periodic Table is arranged by increasing atomic number Increases from left to right, and top to bottom This establishes the periodic law When the elements are arranged in order of increasing atomic #, there is a periodic repetition of their physical & chemical properties Groups- Vertical columns on the periodic table Periods- Horizontal rows of elements- 1-7 • Periodicity- When the elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. Groups…Here’s Where the Periodic Table Gets Useful!! Elements in the same group have similar chemical and physical properties!! (Mendeleev did that on purpose.) Why?? • They have the same number of valence electrons. • They will form the same kinds of ions (have the same ____________). Groups or Families on the Periodic Table Columns are also grouped into families. Families may be one or several columns put together. Families have names rather than numbers. (Just like your family has a common last name.) Representative Elements- All of the Group A Elements 1A-8A Trend # 1 Atomic Radius or Size Group Trend: The Trend: As you move down a group on the periodic table, the atomic radius or size of an atom increases. The Reason: This is because each row adds a new energy level for the electrons. Examples: Which is larger: Mg or Ca Calcium! Which is smaller: F or Br? Fluorine! Period Trend: The Trend: As you move across a period from left to right, the atomic radius or size of an atom decreases. The Reason: This is because of the nucleus getting stronger as you add more protons. This pulls the electrons closer to the nucleus. Examples: Which is smaller: C or F? Fluorine! Which is larger: Ba or Cs? Cs! A Trick to Remembering the Trend: Snowman standing that then falls over: Example: Arrange the following elements in order of increasing size: Mg, Na, Rb, and Cl Cl Mg Na Rb a. K b. S c. Sb d. Ba e. As f. C g. C h. Cs i. Ba j. Si Trend # 2 First Ionization Energy Ionization energy is the amount of energy required to remove an electron from an element in its gaseous state. Li (g) Li+(g) + e- • Ionization energy refers to making positive ions or cations. • To form a positive ion, an element will lose its valence electrons on the outermost energy level. Group Trend: The Trend: As you move down a group on the periodic table, the ionization energy of an atom decreases. Example: Which has smaller ionization energy: Ba or Ca? Barium Which has smaller ionization energy: I or Br? Iodine Period Trend: The Trend: As you move across a period from left to right, the ionization energy of an atom increases. • Examples: Which is lower in ionization energy: C or F? Carbon (C)! Which is higher in ionization energy: Na or Mg? Magnesium (Mg)! The Reason: As an atom gets larger, the valence electrons are further from the nucleus, which means that the nucleus’s pull gets weaker because the electrons are farther away. The weaker pull means that less energy is required to remove the electron. Arrange the following elements in order of decreasing ionization energy: Mg, Na, Rb, and Cl Cl Mg Na Rb A Trick to Remembering the Ionization Energy Trend: Opposite of Atomic Radius: Snowman standing on his head that then falls over: Example: Arrange the following elements in order of increasing ionization energy Mg, Na, Rb, and Cl Rb Na Mg Cl a. K b. S c. Sb d. Ba e. As f. C g. C h. Cs i. Ba j. Si Trend # 3 Electronegativity Electronegativity is the ability of an atom to gain an electron in a chemical bond. Scientists have assigned values for eletronegativity that range from zero to four. The higher values represent the more electronegative elements. Since they are unreactive, the noble gases are not assigned electronegativity values. Group Trend: The Trend: As you move down a group on the periodic table, the electronegativity of an atom decreases. Examples: Which is more electronegative: N or P? Nitrogen (N)! Which has a smaller electronegativity value: F or Br? Bromine (Br)! Period Trend: The Trend: As you move across a period from left to right, the electronegativity of an atom increases. Examples: Which is less electronegative: C or O? Carbon (C)! Which has a greater electronegativity: Ca or K? Ca or K? Calcium (Ca)! The Reason: As an atom gets larger, the valence electrons are further from the nucleus, which means that the nucleus’s pull gets weaker as the electrons move farther away. Smaller elements will be more electronegative because their nucleus is closer to the valence electrons and has a stronger pull on the valence electrons. A Trick to Remembering the Electronegativity Trend: Opposite atomic radius, same as Ionization energy- Snowman standing on his head and then he falls over: Example: Arrange the following elements in order of increasing electronegativity: Cd, Cl, Br, and I Cd I Br Cl Example: Arrange the following elements in order of increasing electronegativity: Cd, Cl, Br, and I Cd I Br Cl a. Cr b. Cl c. N d. Sr e. F f. F g. Ne h. Li i. Be j. Ar Trend # 4 Reactivity - Metals & Nonmetals General Notes Elements in the same group/family have similar chemical and physical properties. What elements would have similar properties to sulfur? answer: O, Se, Te, Po Note: The Representative Elements are those elements within the first two families (Groups I and II on the far left) and the last six families or groups (on the right) of the Periodic Table. Reactivity of Metals Group Trend: The Trend: For metals, the reactivity of metals increases as you move down a group. Examples: Which metal is more reactive: K or Rb? Rubidium (Rb)! Which metal is more reactive: Cu or Au? Gold (Au)! Period Trend: The Trend: For metals, the reactivity of metals decreases as you move across the period from left to right. Examples: Which metal is the least reactive: Ca or Zinc? Zinc (Zn)! Arrange the following metals- Na, Mg, Al in order of increasing reactivity. Al Mg Na The Reason: When metals react, they lose electrons. Metals with low ionization energies are more reactive, or alkali metals are more reactive because they lose electrons more easily. Reactivity of Nonmetals Group Trend: The Trend: For nonmetals, the reactivity of nonmetals decreases as you move down a group. Examples: Which is more reactive: Oxygen or sulfur? Oxygen (O)! Which halogen is the most reactive: F, Cl, Br, or I? Fluorine (F)! Period Trend: The Trend: For nonmetals, the reactivity of nonmetals increases as you move across a period from right to left (excluding the noble gases). Examples: Which is more reactive: O or F? Fluorine (F)! On period 3, which nonmetal is the most reactive? Chlorine (Cl)! The Reason: When nonmetals react, they tend to gain electrons. Nonmetals with higher electronegativities have a stronger pull on electrons and, therefore gain electrons easily. In other words, smaller atoms tend to form negative ions more easily because the nucleus is more likely to attract these electrons. A Trick to Remembering the Trends: Snowman that falls over: Atomic Radius Metal Reactivity A&M likes to stand at football games! A Trick to Remembering the Trends: Snowman standing on his head and then he falls over: • Electronegativity • Ionization Energy Nonmetal Reactivity **EINstein like to do headstands!! Warm-Up What three properties both have the following trend? Decreases as you go down the group and Increases as you go across the period from right to left Trend # 5 Ionic Radii Pg. 10 Comparing Ions In the Same Group: When you are comparing ions of elements in the same group, follow the same trend as the atomic radius or size of the atoms. Examples: Which ion is larger: Mg2+ or Ca2+? Ca2+ larger Which ion is smaller N3- or P3-? N3- smaller Comparing Ions of the Same Element: As an element gains electrons (gets more negative), it will become larger. X2+ X+ x X- X2- Examples: Which ion would be larger: Mg2+ or Mg 2 Mg 2List the ions or atoms in order of increasing size- P+, P2+, P, P-, P3P2+ P+ P P- P3- Comparing Isoelectric Ions (Ions with the Same Number of Electrons) If you are comparing different ions or atoms with the same number of electrons, the largest substance will have the greatest negative charge. Examples: List the following elements or ions from largest to smallest Ne, O2-, F-, Na+, Mg2+ Hint: Atomic radius increases down group, decreases across period O2-, F-, Ne, Na+, Mg2+ Homework: Complete pg. 11 and 12 Metallic Bonds and shielding Metallic Bonds Watch the following video and answer the questions in your notes. http://www.youtube.com/watch?v=3uNETGK_sb4 http://www.youtube.com/watch?v=srxNJ03 W_qM Review Trends 3 factors that affect trends Nuclear size or pull (# of protons in nucleus or location- stronger if smaller atom) # of electron levels or shells- more shells farther away less pull Electron shielding- occurs because of electrons in between them and the nucleus- shields their attraction* think sitting in back of a rock concert- the people (Electrons in other shells) are shielding you from the true enjoyment of the band on stage (the nucleus) because you can’t see through them or hear as well Shielding