unit one: biomolecules exam review sheet
advertisement

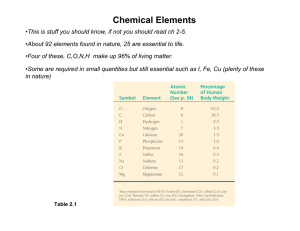
UNIT ONE: BIOMOLECULES EXAM REVIEW SHEET Standards to know: 1. Explain how carbon can join to other carbon atoms in chains and rings to form large and complex molecules. 2. Describe the composition of the four major categories of organic molecules (carbohydrates, lipids, proteins, and nucleic acids). 3. Explain the general structure and primary functions of the major complex organic molecules that compose living organisms. 4. Describe how dehydration and hydrolysis relate to organic molecules. 5. Explain the role of enzymes and other proteins in biochemical functions. 6. Recognize and describe that both living and nonliving things are composed of compounds, which are themselves made up of elements joined by energy-containing bonds, such as those in ATP. 7. Recognize and explain that macromolecules such as lipids contain high energy bonds. Vocabulary terms to know:(ATP, carbohydrate, catalyst, chemical bond, covalent bonds, DNA, dehydration, element, enzyme, hemoglobin, high energy bonds, hormone, hydrolysis, lipid, molecular energy, nucleic acid, protein, protein structure, polymers, RNA, substrate) _____________________________________________________________________________________ General Organic Compounds Questions: (7 questions) 1. What kinds of bonds can carbon form? 2. Why can each carbon atom form four bonds? 3. Which element do all organic compounds contain? 4. What are the four bimolecular? 5. Which kinds of atoms do carbon atoms bond with in biomolecules? 6. What process converts ATP to ADP? 7. Which bond in ATP must be broken to release energy for the cell? 8. In what two biomolecules can animals store energy? 9. Identify the four functional groups. 10. Is alcohol a polar or nonpolar molecule? What functional group is responsible for this characteristic? Carbohydrates: (5 questions) 1. What are the three main groups of carbohydrates? 2. A simple sugar is also known as… 3. A double sugar is also known as… 4. A compound sugar is also known as … 5. List three examples of monosaccharides. 6. List three examples of disaccharides. 7. List three examples of polysaccharides. 8. Which sugars are composed of long chains of monosaccharides? 9. What does a hydrocarbon chain look like? Proteins: (4 questions) 1. Which polymer do amino acids bond to form? 2. What parts of the human body is composed of protein? 3. How do enzymes affect chemical reactions in your body? Lipids: (7 questions) 1. What are the three parts of a phospholipid? 2. Are lipids polar and nonpolar? 3. What can lipids dissolve in? (hint: water is a polar molecule) 4. How are saturated fatty acids different from unsaturated fatty acids? 5. What four groups of molecules are examples of lipids? Nucleic Acids: (2 questions) 1. What are the two types of nucleic acids? 2. Nucleic acids are very long polymers. What are the monomer units for nucleic acids called?