Unit Four: Chemical Formulas
advertisement
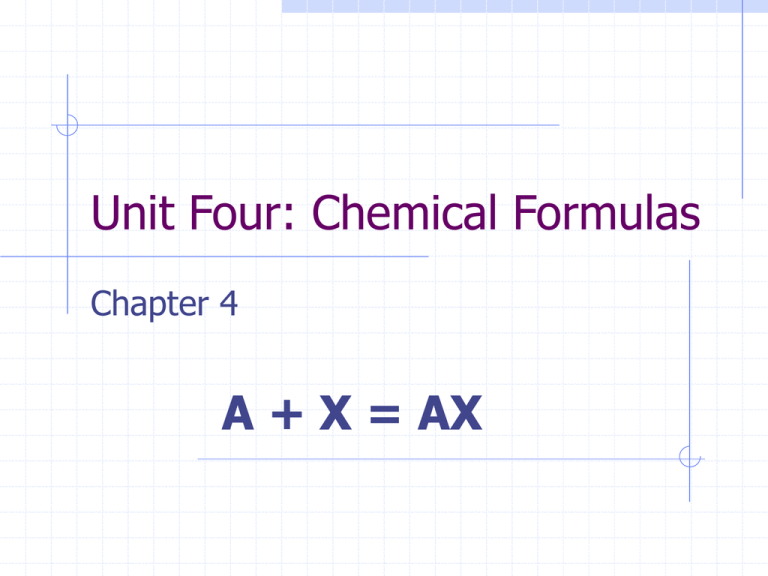
Unit Four: Chemical Formulas Chapter 4 A + X = AX Unit Objectives Upon competition of this unit, you will be able to: List names and symbols of common elements Differentiate between chemical symbols and formulas Differentiate between subscripts and coefficients Given a formula, state the number of atoms of each element present Define compound, ion, polyatomic ion, and oxidation number List the names, symbols, and oxidation numbers or charges of common ions Objectives Continued List the common elements that normally occur as diatomic molecules Write formulas for chemical compounds using oxidation numbers Distinguish atoms, ions, and molecules Define, recognize, and give examples of binary compounds Name compounds from their chemical formula Recognize and give examples of compounds containing polyatomic ions Differentiate between molecular and empirical formulas Define, recognize, and give examples of a formula unit Names and Symbols Chemical Symbols are shorthand representations of the elements See Table 4-1 Symbols usually come from the first letter of the name, or in some cases the Latin version of the name Ac = Actinium Al = Aluminum I = Iodine Fe = Iron Chemical Formulas A Chemical Formula is a combination of symbols that represents the composition of a compound A formula shows two things 1. 2. Elements Present Relative number of atoms of each element in the compound H2O = Hydrogen and Oxygen present, 2 Hydrogen's and 1 Oxygen Oxidation Number A charged atom is called an ion An ion made of more than one atom is called a polyatomic ion When a single atom takes on a charge, that charge is known as the oxidation number of the atom See table 4-3 and 4-4 on page 65 Ionic Compounds Table salt, NaCl, is an ionic compound Table 4-3 shows that Na has a 1+ charge and Cl has a 1- charge They combine in a 1:1 ratio to give a net charge of 0 1 + (1-) = 0 Therefore, the formula is NaCl An ionic compound is composed of charged particles Neutral atoms combine to form molecules. Diatomic Molecules A diatomic molecule is composed of two atoms of the same element Seven common elements occur as diatomic molecules You will need to memorize these They are: Chlorine (Cl2), Hydrogen (H2), Nitrogen (N2), Oxygen (O2), Fluorine (F2), Bromine (Br2), and Iodine (I2) Any time these element exist on their own, they will be found as diatomic molecules I.e., You will never find just one hydrogen floating around Practice Write the formula for a compound of Hydrogen and Bromine Write the formula for a compound of Ca and Cl Write the formula for a compound of Aluminum Hydroxide Practice Write the formula for a compound of Hydrogen and Bromine HBr Write the formula for a compound of Ca and Cl CaCl2 Write the formula for a compound of Aluminum Hydroxide Al(OH)3 Points to remember If an ion has a roman numeral after its symbol, that means that it has the charge indicated by the roman numeral Pb(II) = Lead 2+ Cr(III) = Chromium 3+ Use parenthesis when you have more than one polyatomic ion in a compound Mg(CN)2 Assignment Complete Exercises 1-3 on page 67 of the text Worth a possible 10 points Due: Tomorrow Naming Compounds Many compounds have two names, common and chemical NaHCO3 is sodium hydrogen carbonate, but is also known as baking soda We will use systematic naming Naming Binary Compounds Compounds containing only two elements are called binary compounds To name a binary compound 1. 2. Write the name of the element having a positive charge Then add the name of the negative element, modifying the ending to -ide Example: HBr = Hydrogen Bromide Naming Binary Compounds cont. Some elements have more than one possible charge When we name these, we use roman numerals after the name of the element to show its oxidation number NO = Nitrogen (II) Oxide (see table 4-6) Prefixes In an older naming system, prefixes were used to indicate the number of atoms (table 4-7) Prefix Mono Di # of atoms 1 2 Tri Tetra Penta 3 4 5 Hexa Hepta Octa 6 7 8 Common Acids The names of the common acids do not follow the rules of systematic naming You will have to memorize the names and formulas of the common acids See table 4-9 on page 69 of the text Organic Compounds Organics are named using a different set of rules Count the number of carbon atoms in a chain The beginning of the name will be in table 4-10 Then add suffix –ane Example: C6H14 Hexane Assignment Complete Exercises 4-8 on page70 Worth a possible 10 points Due: Molecular and Empirical Formulas The formulas for compounds that exist as molecules are called molecular formulas Hydrogen peroxide is H202 Shows actual number of atoms in the molecule An empirical formula indicates the simplest whole-number ratio of atoms or ions in a compound For hydrogen peroxide, the empirical formula would be HO Coefficients The formula of a compound represents a definite amount of that compound, this is called a formula unit One formula unit of water is H2O If you had two formula units of water, it would be written as 2H2O The “2” is called a coefficient Remember from algebra, if you have 2 x’s (or x+x), you would say you had 2x Coefficients are used to represent the number of formula units Assignment Complete Exercises 9-10 on page 72 Worth a possible 10 points Due: By the end of class Chapter Review Complete the following questions on pages 73-75 13-19, 22-40, 44, 47, 48, 49, and 50 Worth a possible 25 points Due before the test





