10xylene
advertisement

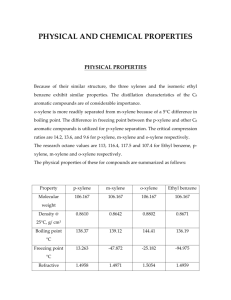
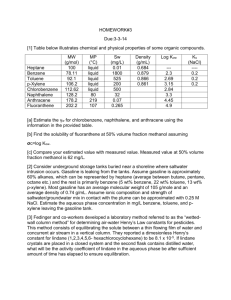
SATHYA P NARAYANAN SAURABH CHANSOLIYA SAURAV JAYAN B130512CH B130524CH B130196CH CONTENTS Raw materials Chemical reactions Properties Flow diagram Process descriptions Engineering problems Applications Alternate processes for production RAW MATERIALS Petroleum Naphtha is the main raw material Coke Hydrogen Toluene CHEMICAL REACTIONS Xylenes undergo electrophilic substitution reactions in the same manner as toluene. Upon oxidation with KMnO4 or K2Cr2O7, Xylenes form corresponding dicarboxylic acids. M-xylene to P-xylene PROPERTIES CHEMICAL PROPERTIES: Reactions involving the position of the alkyl substituents: These reactions include isomerization, disproportionation and dealkylation. Acids catalyze the interconversion of the three-xylene isomers. Xylenes isomerize to near equilibrium levels in a hydrogen fluoride – boron trifluoride system with low boron trifluoride concentrations. Isomerization at lower temperatures produces more p-xylene and o-xylene. PHYSICAL PROPERTIES Because of their similar structure, the three xylenes and the isomeric ethyl benzene exhibit similar properties. The distillation characteristics of the C8 aromatic compounds are of considerable importance. o-xylene is more readily separated from m-xylene because of a 5°C difference in boiling point. The difference in freezing point between the p-xylene and other C8 aromatic compounds is utilized for p-xylene separation. The critical compression ratios are 14.2, 13.6, and 9.6 for p-xylene, mxylene and o-xylene respectively. The research octane values are 113, 116.4, 117.5 and 107.4 for Ethyl benzene, pxylene, m-xylene and o-xylene respectively. FLOW DIAGRAM Meta Xylene Production P-Xylene Production Process Description Catalytic Reforming Catalytic reformate is the major source of xylene, accounting for approximately 95 percent of the xylene production capacity feedstocks.1,3 Catalytic reforming involves the catalytic dehydrogenation of straight-run light naphtha in the presence of hydrogen (which reduces coke formation) to yield a mixture of aromatic hydrocarbons (e.g., benzene, toluene, and the xylenes. P-Xylene is produced by catalytic reforming of petroleum naphtha as part of the BTX aromatics (benzene, toluene and the xylene isomers) extracted from the catalytic reformate. The P-Xylene is then separated out in a series of distillation, adsorption or crystallization and reaction processes from the mxylene, o-xylene and ethylbenzene. Its melting point is the highest among this series of isomers, but simple crystallization does not allow easy purification due to the formation of eutectic mixtures. It is also highly flammable. Problems Atmospheric releases of xylenes are primarily as fugitive emissions from industrial sources (e.g., petroleum refineries, chemical plants); as emissions in automotive exhausts; and as a result of volatilization from their use as a solvent. Due to the high volatility of xylenes, most environmental releases partition to the atmosphere. Xylenes are moderately mobile in soil, where they may be adsorbed. Xylenes may leach into groundwater, where they can persist for several years. Xylenes are rapidly transformed by photo-oxidation in the troposphere, and can participate in the formation of groundlevel ozone. Xylenes are stable to hydrolysis and oxidation in the aquatic environment. Applications Terephthalic acid and related derivatives p-Xylene is the principal precursor to terephthalic acid and dimethyl terephthalate, both monomers used in the production of polyethylene terephthalate (PET) plastic bottles and polyester clothing. 98% of p-xylene production, and half of all xylene, is consumed in this way. o-Xylene is an important precursor to phthalic anhydride. The demand for isophthalic acid is relatively modest so m-xylene is rarely sought (and hence the utility of its conversion to the o- and p-isomers). Xylene is used as a solvent. In this application, the mixture of isomers is often referred to as xylenes or xylol. Solvent xylene often contains a small percentage of ethylbenzene. Like the individual isomers, the mixture is colorless, sweet-smelling, and highly flammable. Areas of application include the printing, rubber, and leather industries. It is a common component of ink, rubber, and adhesive. In thinning paints and varnishes, it can be substituted for toluene where slower drying is desired, and thus is used by conservators of art objects in solubility testing. Cleaning agent Similarly it is a cleaning agent, e.g., for steel, silicon wafers, and integrated circuits. In dentistry, xylene can be used to dissolve gutta percha, a material used for endodontics (root canal treatments). In the petroleum industry, xylene is also a frequent component of paraffin solvents, used when the tubing becomes clogged with paraffin wax. For similar reasons, it is often the active ingredient in commercial products for ear wax (cerumen) removal. Laboratory uses It is used in the laboratory to make baths with dry ice to cool reaction vessels, and as a solvent to remove synthetic immersion oil from the microscope objective in light microscopy. In histology, xylene is the most widely used clearing agent. Xylene is used to remove paraffin from dried microscope slides prior to staining. After staining, microscope slides are put in xylene prior to mounting with a coverslip. Precursor to other compounds Although conversion to terephthalic acid is the dominant chemical conversion, xylenes are precursors to other chemical compounds. For instance chlorination of both methyl groups gives the corresponding xylene dichlorides (bis(chloromethyl)benzenes) whilst monobromination yields xylyl bromide, a tear gas-agent used in World War I. NEWER ALTERNATIVES FOR PRODUCTION OF XYLENE CRYSTALLISATION TECHNOLOGY This process is characterized by the use of adsorption technology to produce a medium-purity paraxylene feedstock stream that goes into a low-temperature crystallization plant to produce crystals. These crystals are centrifuged and melted to produce high purity paraxylene. . TOLUENE ALKYLATION A relatively new development is the toluene alkylation process, in which p-xylene is obtained by reacting toluene and methanol with little benzene by-product. In the pxylene recovery field, adsorptive separation has been the dominant process worldwide, although crystallization technology has attracted renewed interest. In addition to updated configuration and processing schemes, which result in higher unit efficiency, modern crystallization plants use more reliable and larger-scale equipment. New p-xylene crystallization capacity additions have been made in combination with the paraselective toluene disproportionation technology Thank You.