Separation by pervaporation of para and meta xylene in the... by Randi Wright Wytcherley
advertisement

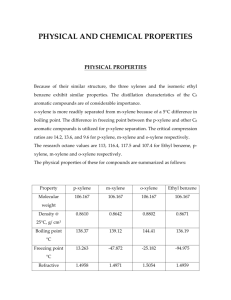
Separation by pervaporation of para and meta xylene in the presence of tetrabromide by Randi Wright Wytcherley A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by Randi Wright Wytcherley (1987) Abstract: The separation of para and meta xylene was investigated by pervaporation in the presence of CBr across a polypropylene membrane. Data was collected for experimental runs varying percent p-xylene at 10%, 30%, 50%, 70%, and 90% in the para and meta xylene mixture, for temperatures of -20°C, 5°C, 22°C, 50°C, and 60°C, with zero, 10, and 24 mole % CBr4 added to the xylene mixture. Evaluation of the data determined if the degree of separation was affected by any of the variables. The results were compared using a calculated separation factor which is somewhat similar to the relative volatility in distillation. Since p-xylene is the more volatile of the two isomers, and the membrane used is selective for p-xylene, it was chosen as the basis when calculating the separation factor. When CBr4 was added to a mixture of para and meta xylene, a solid complex was formed between the CBr and the p-xylene under certain conditions. An association between the CBr4 and p-xylene was present in the liquid phase under certain conditions. The complex or association formation was dependent on the concentration of p-xylene in the xylene mixture, the amount of CBr4 added, and the temperature. Both the solid complex and the association tied up the p-xylene in the feed and reduced the amount of p-xylene available to permeate through the membrane. The amount of m-xylene which was available to permeate through the membrane was unchanged. Therefore, more m-xylene permeated through the membrane than p-xylene and so the m-xylene was concentrated in the product. The result was an increased separability of the pervaporation process for m-xylene. The greatest separation occurred at -20°C with 90% p-xylene in the para and meta xylene mixture, and 24 mole % CBr4 added to the xylene mixture. These conditions yielded a separation factor for p-xylene of 0.05. The inverse of the p-xylene separation factor is the separation factor for m-xylene, so under these conditions, the resulting m-xylene separation factor was 20. In general, the separation of para and meta xylene by pervaporation can be significantly enhanced when 24 mole % CBr4 was added to the feed side of the membrane with high p-xylene content in the feed at temperatures between 5°C and -20°C. SEPARATION BY PERVAPORATION OF PARA AND META XYLENE IN THE PRESENCE OF CARBON TETRABROMIDE by Randi Wright Wytcherley A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Chemical Engineering MONTANA STATE UNIVERSITY Boz eman, Montana May 1987 MAIN LIB. V37? l/9?Y ii APPROVAL of a thesis submitted by Randi Wright Wytcherley This thesis has been read by each member of the thesis committee and has been found to be satisfactory regarding content, English usage, format, citation, bibliographic style and consistency, and is ready for submission to the College of Graduate Studies. Date Chairperson, Graduate Committee Approved for the Major Department Date Approved for the College of Graduate Studies Date Graduate Dean iii STATEMENT OF PERMISSION TO USE In presenting this thesis requirements for a in partial' fulfillment of the master's degree at Montana State University, I agree that the Library shall make it available to borrowers under rules from this thesis are of the Library. Brief quotations allowable without special permission, provided that accurate acknowledgment of source is. made. , Permission for extensive of this thesis may be granted his absence, by the Dean of quotation from or reproduction by my major professor, or in Libraries when, in the opinion of either, the proposed use of the material is for scholarly purposes. for Any copying or use of the material in this thesis financial gain permission. Signature. Date_____ >5H I an shall not be allowed without my iv ACKNOWLEDGMENTS The author would like to the Chemical Engineering University, for their and encouragement research by appreciated. my thank the faculty and staff of Department guidance given advisor. The author P. Mundy and doctorate Chemistry Department at and also F.P. the course State The advice of this McCandless, is greatly wishes to thank Dr. Bradford candidate Montana Montana assistance. throughout Dr. at Mr. Dave Barnekow of the State University for their assistance with the infrared spectroscopy. V TABLE OF CONTENTS Page APPROVAL.............. ......... . ...... ..... ii STATEMENT OF PERMISSION TO U S E .............. xii ACKNOWLEDGMENTS........... iv TABLE OF CONTENTS........ v ABSTRACT. . .................... ....... ‘........ INTRODUCTION.......................... Separating the Xylenes...... . . . Pervaporation.................... Previous Related W o r k .... ...... Selective Complex Formation with Aromatics................................ Separation Factor........................ viii xiv I w LIST OF FIGURES............... vii m LIST OF TABLES................................ 7 11 RESEARCH OBJECTIVES.... ..................... 13 EXPERIMENTAL APPARATUS AND PROCEDURE..... . . 14 Pervaporation Test Ce l l...... Constant Temperature Bath and Circulating System....... Vacuum System and Cold Tra p s............ Product Analysis.............. Experimental Procedure........ RESULTS AND DISCUSSION............ Discussion of Results...... P-Xylene, m-Xylene and CBr^ System..... 14 17 18 18 18 21 38 40 vi TABLE OF COMTENTS— Continued Page Formation of CBr • p-xylene Association in Liquid Phase......................... Carbon Tetrabromide in Product.......... Advantage of Using Pervaporation Process............... .............* . . . . Effect of Complexing Agent on Fl u x ..... 54 55 CONCLUSIONS................................... 59 SUGGESTIONS FOR FUTURE RESEARCH. ............. 61 LITERATURE CITED......... 62 APPENDIX....... 65 Cross Plots for Figures 13 through 17... 52 53 65 vii LIST OF TABLES Table Page 1. Physical Data for the C g Aromatics.......... 2 2. Various Mole % CBr Added to Xylene Feed Mixture to Complex with the p-Xylene........ 19 Initial Crystallization Temperatures for Binary Mixtures of p-Xylene and m-Xylene___ 39 3. 4. Melting Points for the CBr • p-xylene complex at High Concentrations of p-Xylene in the Xylene Feed Mixture.......................... 53 5. CBr^ Present in the Product of the 30/70 Ratio (para to meta) Xylene Run with 10 mole % CBr^................................ 53 Flux for the Runs at O r I O r and 24 Mole % CBr 57 6. viii LIST OF FIGURES Figure 1. Initial crystallization temperature of binary mixtures of carbon tetrachloride and a xylene with the eutectic points circled...................... Page g 2. Initial crystallization temperature of binary mixtures of carbon tetrabromide and a xylene with the eutectic points circled...................................... 10 3. Pervaporation Equimpment Diagram........... 15 4. Pervaporation Test C e l l..................... 16 5. Separation factors vs temperature at varying percent p-xylene in the para and meta xylene mixture across a polypropylene membrane with no CBr4 added....................... 22 6 . Percent p-xylene in the product as a function of the % p-xylene in the feed at varying temperatures with no CBr added.......... ..................... ; ....... 23 7. Separation factor vs temperature for 10% p-xylene and varying % CBr additions...................... ? 24 ix LIST OF FIGURES— Continued Figure Page 8 . Separation factor vs temperature for 30% p-xylene and varying % CBr additions........................... 25 9. Separation factor vs temperature for 50% p-xylene and varying % CBr additions.... ................. t ............ 26 10. Separation factor vs temperature for 70% p-xylene and varying % CBr additions........ 27 11. Separation factor vs temperature for 90% p-xylene and varying % CBr additions...... ............... * ...... ...... 28 12. Percent p-xylene in product as a function of % p-xylene in feed with 10 and 24 mole % CBrif added at 6 0 °C.................. 29 13. Percent p-xylene in product as a function of % p-xylene in feed with 0 and 24 mole % CBrjf added at 50°C......... 30 14. Percent p-xylene in product as a function of % p-xylene in feed with 0 and 24 mole % CBr added at 22 0C .................. 31 15. Percent p-xylene in product as a function of % p-xylene in feed with 0 and 24 mole % CBrjf added at 5 0C ................... 32 4 X LIST OF FIGURES— Continued Figure Page 16. Percent p-xylene in product as a function of % p-xylene in feed with 0 and 24 mole % CBr^ added at -200C ................. 33 17. Freezing point diagrams for mixtures of p-xylene, m-xylene, and CCl , and p-xylene, m-xylene, and CBr^.........7 ................ 42 18. Mole % CBr present in liquid phase as a function of p-xylene concentration in the para and meta xylene mixture at 2 2 0C with an initial concentration of CBr at 24 raole%........................ T ........... 46 19. Freezing point diagram for mixtures of p-xylene, m-xylene, and CBr^............ 48 20. Freezing point diagram for mixtures of p-xylene, m-xylene, and CBr4 ........... . 51 21. Average flux for pervaporation process with 0, 10, and 24 mole % CBr4 added...... 56 22. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 10% p-xylene in the xylene mixture with no CBr 23. Separation factors (a) produced as a function of temperature from the pervapdrat pervaporation separation of a feed mixture containing 30% p-xylene in the xylene mixture with no CBr added.............. .........................? 67 xi LIST OF FIGURES— Continued Figure Page 24. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 50% p-xylene in the xylene mixture with no CBr added..................................... . .? 68 25. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 70% p-xylene in the xylene mixture with no CBr added....................................... ? 69 26. Separation factors (ot) produced as a function of temperature from the pervaporation separation of a feed mixture containing 90% p-xylene in the xylene mixture with no CBr added....................................... ? 70 27. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 10% p-xylene in the xylene mixture with 10 mole % CBr^ added................................... 71 28. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 30% p-xylene in the xylene mixture with 10 mole % CBr added............. ..................... 72 4 29. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 50% p-xylene in the xylene mixture with 10 mole % CBr^ added........................... ....... 73 xii LIST OF FIGURES— Continued Figure Page 30. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 70% p-xylene in the xylene mixture with 10 mole % CBr4 added................................... 74 31. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 90% p-xylene in the xylene mixture with 10 mole % 75 CBr added................................... 32. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 10% p-xylene in the xylene mixture with 24 mole % CBr4 added................................... 76 33. Separation factors (ot) produced as a function of temperature from the pervaporation separation of a feed mixture containing 30% p-xylene in the xylene mixture with 24 mole % CBr added.................. ................ 77 34. Separation factors (ot) produced as a function of temperature from the pervaporation separation of a feed mixture containing 50% p-xylene in the xylene mixture with 24 mole % 7B CBr added.................. ................ 35. Separation factors (ot) produced as a function of temperature from the pervaporation separation of a feed mixture containing 70% p-xylene in the xylene mixture with 24 mole % CBr4 added................................... 79 xiii LIST OF FIGURES— Continued Figure . Page 36. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 90% p-xylene in the xylene mixture with 24 mole % CBr added............. ............... . 80 xiv ABSTRACT The separation of para and. meta xylene was investigated by pervaporation in the presence of CBr across a polypropylene membrane. Data was collected for experimental runs varying percent p-xylene at 10%, 30%, 50%, 70%, and 90% in the para and meta xylene mixture, for temperatures of -200Cj 5°C, 22°C, 50°C, and 60°C, with zero, 10, and 24 mole % CBr^ added to the xylene mixture. Evaluation of the data determined if the degree of separation was affected by any of the variables. The results were compared using a calculated separation factor which is somewhat similar to the relative volatility in distillation. Since p-xylene is the more volatile of the two isomers, and the membrane used is selective for p-xylene, it was chosen as the basis when calculating the separation factor. When CBr^ was added to a mixture of para and meta xylene, a solid complex was formed between the CBr and the p-xylene under certain conditions. An association between the CBr4 and p-xylene was present in the liquid phase under certain conditions. The complex or association formation was dependent on the concentration of p-xylene in the xylene mixture, the amount of CBr added, and the temperature. Both the solid complex and the association tied up the px y Iene in the feed and reduced the amount of p-xylene available to permeate through the membrane. The amount of m-xylene which was available to permeate through the membrane was unchanged. Therefore, more m-xylene permeated through the membrane than p-xylene and so the m-xylene was concentrated in the product. The result was an increased separability of the pervaporation process for m-xylene. The greatest separation occurred at -20°C with 90% pxylene in the para and meta xylene mixture, and 24 mole % CBr4 added to the xylene mixture. These conditions yielded a separation factor for p-xylene of 0.05. The inverse of the p-xylene separation factor is the separation factor for m-xylene, so under these conditions, the resulting m-xylene separation factor was 2 0 . In general, the separation of para and meta xylene by pervaporation can be significantly enhanced when 24 mole % CBr4 was added to the feed side of the membrane with high pxylene content in the feed at temperatures between 5 0C and -20°C. I INTRODUCTION Xylenes are important of plastics and dyes. ingredients But in for the manufacture order for the xylenes to be useful they must be high in purity. Xylenes are produced in petroleum refining as a by product from catalytic reforming. They are produced as meta (m ) , and para a (p ), mixture of the isomers, ortho (o ) , along with ethylbenzene in varying compositions depending on the conditions in the reformer. The structure of these isomers are: ethylbenzene o-xylene The boiling points and along with the combinations emphasize presents. the of m-xylene melting relative the points for the isomers volatilities isomers challenging p-xylene are shown separation of the in Table problem various that I to this 2 Table I. Physical Data for the C q Aromatics -ing points and me.Lting points ISOMER B P ethylbenzene o-xylene m-xylene p-xylene b. 136.2 144.4 139.1 138.4 Df C C C C the C arcDmatics M P3 -94.4 -25.2 -47.9 13.3 C C C C Relative volatility for binary combinations (I) RELATIVE BINARY MIXTURE VOLATILITY ethylbenzene p-xylene + m-xylene + ethylbenzene ethylbenzene p-xylene + + o-xylene o-xylene o-xylene + m-xylene + p-xylene m-xylene 1.34 1.28 1.22 1.08 1.06 1.01 Separating the Xylene Isomers In order for the isomers to be effectively separated by distillation the relative 1.0. If the relative separation of the volatility volatility compounds is than 1 .0 , listed in Table lb is concentrated relative volatility is between zero in the product. lb be greater than equal to 1.0, then no occur. volatility is greater component listed in Table must then If the relative the first component in the product. If the and 1.0 then the second would be the one concentrated For economical separation by distillation a relative volatility of about 1.25 is necessary (2). The ethylbenzene and ortho xylene are usually separated from the mixture number of plates of isomers (3). The by distillation with a large challenge comes with the 3 separation of volatility the of para their and. meta binary mixture therefore separation by normal as it would require an xylenes. is The relative equal to 1 .0 1 , distillation is not feasible extremely large number of stages at very high reflux ratio. Several processes separation of para based on have and meta crystallization presence.of HF-BF3 (5). This rotary allows operation. the Some processes are extraction in the the most successful process is based on adsorption by a that which for Parex process, which was developed zeolite in a process valve solvent Currently, separation method is the by U O P , Inc. developed xylene. or (4). been utilizes a complex and expensive simulation of a moving bed In principle, this adsorption system operates as a chromatographic column. The xylene mixture is fed to the unit as a pulse followed by a unit alternatively outlet is collected pulse of the d e sorbent. as The m-xylene and desorbent or p-xylene and desorbent (6 ). More recently, principle, has been another developed type of zeolite with an in order to of the improve by Asahi based on (7). the same A different appropriate desorbent fluid is used the displacement chromatography effect separation. Both selective adsorption of a on zeolite. process, processes liquid are based on the mixture of xylene isomers In the above processes the separation of liquid mixtures of the xylenes has been carried out by adsorption 4 on zeolite particles. same separation continues in in this Recently Carra the gaseous area since et al. proposed the phase the (8 ). Research commercial methods currently used are very complicated and energy intensive. Pervanoration Pervaporation is a membrane fractionate liquid mixtures. processes, membrane processes separate it into two product contact In with Like take side of other feed separation stream and In one the target the other the target species pervaporation one a streams. species is concentrated and in is depleted. separation process used to a a liquid feed is placed in nonporous membrane. The components of the feed mixture pass through the membrane and leave the downstream side as a vapor. separation is the difference in chemical potential between the liquid and the vapor phases. permeation may be The driving force for The driving force for the attributed to the pressure and concentration differences across the membrane. There are a few permeating molecules theories during as surface in contact I. the migration through the body of the permeating material et Solution with at the behavior of the pervaporation. theory was proposed by Binning three step process: to al. (9) which involves a of liquid into the film liquid the film; the One possible charge mixture; 2. 3 . vaporization of downstream interface where 5 the permeate is immediately swept away. the permeation rate and separation predicted by the components in the permeation feed of the mixture cannot be rates since With this theory, the of the individual membrane structure may change due to swelling (1 0 ). Another proposal as to molecules is that in that the screen. membrane the which acted behavior of the permeating Michaels et al. as a simple (11) postulated molecular sieve or In this case the permeation rate of a mixture could be predicted from the permeation rates of the individual components. The pervaporation process uses nonporous membranes. The permselectivity of these membranes comes from properties inherent to the membrane material. defined as the rates of flux of the two isomers under equal partial pressure driving forces generally depends on the diffusivity the species being separated (12).. in the The permselectivity and the solubility of the membrane material. permselectivity is best when there either the diffusivity or Permselectivity is The is a large difference in solubility of the permeating species and the rejected species. Diffusivity is dependent on molecular size and shape as well as the mechanical properties of the polymer. the chemistry of the also very important. polymer chemical interactions affect is the However, The solubility of the species 6 in the membrane. In principle, the more soluble a species, the higher the permeability (13). Pervaporation can sometimes separation of certain mixtures separate. Liquid mixtures have very close vapor chemical membrane, on interactions a which which helpful in the are otherwise hard to form azeotropes or which pressures are virtually impossible to separate by conventional separation depends prove distillation. the mechanical between successful Since pervaporation properties the molecules separation of close and the and the boiling components is sometimes possible. Previous Related Work Selective complex formation used which forms a complex component. occurs when an agent is between the agent.and a specific The formation of this complex can increase or at least change the selectivity of membrane processes. Work has been done past where the complexes (14). membrane at Montana State University in the itself was modified using Werner The result was an increased selectivity for the target species. Recently, work has been done in coupled transport or facilitated transport which somewhat overcomes the membranes inability to make clean separations (15). from the membrane. incorporation This carrier of a forms specific a Selectivity comes carrier within the complex with the target I species on the feed side diffuses through the of the membrane; the complex then membrane and the target species is released on the product side of the membrane. Another approach is based nature of the target species on the idea of changing the in membrane will be either more or the feed solution so the less permeable to it. has been done with reverse osmosis (RO) where a formed resulting in a The RO membrane is less to the heavy permeable the to the higher molecular target species. ultrafiltration heavy metal ions (17). up complex was higher molecular weight species (16). weight complex than to has been applied This Complexing metal ions, ultrafiltered, the metal for removal of toxic agents were used to tie the complex The same idea solution was then broken and the complexing agent recovered for re-use. Selective Complex Formation with Aromatics Previous work indicated the complexes between discussed formation Cg, by of Cg, tetrahalogenated methanes. Egan solid Cg , An and et al. (18) molecular C iq temperature binary mixtures of single eutectic indicates no of CCl^ observed complex was in with equimolal complex is formed initial and addition aromatics between CCl^ and p-xylene which freezes at -3.90C. shows the has the the formed. Figure I crystallization for the xylene case isomers (19). of meta A xylene Other tetrahalogenated 8 - 25.00 P-XYLENE 50.00 LU O Q^O - CZ - 100.00 -7 5 . 0 0 M-XYLENE TEMPERATURE “C 25.00 o o 0 .00 20 . 00 4 0 . 00 60.00 8 0 . OO 100. OO MOLE % AROMATIC Figure I. Initial crystallization temperature of binary mixtures of carbon tetrachloride and a xylene, with the eutectic points circled. 9 methanes were also with p-xylene. found One of to form solid addition compounds these was form an equimolal complex that shows the temperatures occurred for the isomers (20). freezes at 53.30C. where binary Carbon only p-xylene. initial mixtures of as Figure 2 crystallization CBr^ tetrabromide with both of the xylene isomers in forming complexes CBr4 , which was found to and the xylene had complex formation and so was not as selective CCl4 which formed a complex with Complex formation is indicated for both para and meta xylene with CBr4 for each binary mixture. since two eutectics are present The eutectic points are circled in Figures I and 2. This research project involves the separation of the para and meta xylene isomers in the presence of CBr4 . Under certain conditions a molecular complex is formed between the CBr4 and the p-xylene. complex of CBr4 and the amount of The p-xylene p-xylene presence in which through the polypropylene xylene present in the is available to permeate This decrease in the permeate results in less p- product. available to permeate through the molecular the feed solution reduces membrane. amount of p-xylene available to of The amount of m-xylene the membrane is not affected. The result is an increase of separability of the process for m-xylene, with more m-xylene in the feed. CBr4 was appearing chosen as in the product than the complexing agent 10 -50.00 0.00 P-XYLENE - 100.00 M-XYLENE TEMPERATURE 0C 5 0 . 00 O O d 0 . 00 20 . 00 40.00 60.00 8 0 . 00 1 0 0 . 00 MOLE % AROMATIC Figure 2. Initial crystallization temperature of binary mixtures of carbon tetrabromide and a xylene with the eutectic points circled. 11 since it has a higher temperature of complex formation and. it is less toxic than CCl4 . Due to the limited availability of membrane material, a Dupont polypropylene membrane which was on hand was used. \ Since the complexing agent tied membrane was selective to the para to meta xylene up the p-xylene while the the p-xylene, the separability of varied greatly, depending on which effect was greater. Separation Factor The separation factor (<x) is calculated using the following equation: cc = y( 1-x) X(l-y) ' <x = separation factor with respect to p-xylene y = fraction p-xylene in product x = fraction p-xylene in feed This equation gives a separation factor in terms of pxylene. The separation relative volatility factor is greater transfer of p-xylene the membrane would be separation factor is factor in is somewhat similar to the distillation. than 1 .0 , across there the selective between 0.0 If the separation would be a greater membrane than m-xylene or to the and p-xylene. If the 1.0 the membrane is selective for the m-xylene. In the research, the interest lay in decreasing the of feed, which could amount diffuse p-xylene available in the through the membrane, by 12 completing it amount of with m-xylene the carbon available tetrabromide. to permeate Since the through the membrane was unchanged, the m-xylene was concentrated in the product. This would appear as a lower separation factor or an increased selectivity of the process for m-xylene. 13 RESEARCH OBJECTIVES This research was conducted to determine if the addition of carbon tetrabromide as a completing agent would change the pervaporation separation of a liquid mixture containing para effects and meta xylene. tetrabromide to the The xylene determine if the p-xylene The effect of mixture adding carbon were examined to available to permeate through the membrane decreased, thereby product. feed of increasing concentration the m-xylene in the of the CBr and p4 xylene along with the c temperature was investigated for both the complexing and the separability. 14 EXPERIMENTAL APPARATUS AKfD PROCEDURE A pervaporation experiments for Figure 3 test separation illustrates concentration cell of of the liquid temperature the feed was was bath to conduct the and meta xylene. setup. placed The in known (B) constantly mixed (E). and controlled the temperature of used para equipment pervaporation cell where it constant was circulation the system the The (A) liquid feed in the cell. The vacuum system (D) pulled the vapor product into the cold traps (C) where it was crystallized using liquid nitrogen as the cooling medium. When a sufficient amount of product had been collected in Cl„ the vacuum pump was turned off and the product was weighed on a Mettler P1200 balance to ±0.01 grams and analyzed using a gas chromatograph (GC). Pervaporation Test Cell The pervaporation Figure 4. The test test cell cell was is shown constructed in of detail in two 8 cm diameter stainless steel flat face flanges made by modifying a large pipe union. stainless steel n u t . These were held together by a large Inside the bottom flange was space for a 3.8 cm diameter perforated disc and filter paper which was used to support the membrane. This flange was 15 do— i A. Controlled Temperature Bath and Circulating System B. Pervaporation Cell C. Cold Traps D. Vacuum Pump E. Mixer Figure 3. Pervaporation Equimpment Diagram. 16 BULK LIQUID T==IT.!:.. MEMBRANE FILTER PAPER IPERFORATED PLASTIC DISC RINGS I_ I BULK VAPOR ^ r f. ™ Figure 4. Pervaporation Test Cell. 17 connected to the vacuum 1/2 teflon tubing. system with swedgelock fittings and The top flange had threads to match the large nut and an open cavity feed was constantly to hold the liquid feed. mixed to avoid any This concentration gradients against the membrane. The Dupont polypropylene, Clysar® 350P-1A3, 20ymr was membrane flanges and the large nut leakage. held between the two tightened securely to prevent any Two viton "0" rings were offset on the flanges to provide the protection against leakage. The liquid feed was where the mixer placed simulated mixer consisted of a' a in the upper flange cavity perfectly converted mixed system. The variable speed drill with a variac power control. Constant Temperature Bath and Circulating System The constant temperature Circulator made by the capability mixture held Masterline of at bath a Forma circulating specified an was a 2095 Bath and Scientific. This had ethylene temperature. glycol/water The ethylene glycol/water mixture was circulated through insulated tubing into insulated casing which flanges. The temperature surrounded both upper and lower of the liquid feed was monitored by a 2108A digital thermometer (manufactured by Fluke). feed could be ±1°C. controlled to The temperatures ranged on the temperature desired. a The temperature usually within from -230C to 6 1 0C depending 18 Vacuum System and Cold Traps Two cold finger Pyrex condensers series to the vacuum line and glass fittings and spring the permeate while other diffusion from the vacuum condensers were placed nitrogen. A two stage a a mercury used to prevent hack and mercury manometer. The dewer flask containing liquid duo-seal capacity provided a vacuum of monitored by One condenser collected was pump in connected in to the test cell using ground clamps. the were vacuum 0.5 U-tuhe pump running at pm Hg. monometer The vacuum was and at times a McCloud guage. Product Analysis A quantitative analysis of the permeate was found using a Varian Aerograph Series 1400 Sargent-Welch recorder, model with Bentone 34 para and meta modified xylene gas SRG. with chromatograph The column was packed diisodecylphthalate for the analyses, but for' the quantitative determination of CBr^, SR-30 packing was required. packing is also as boiling point known analyses usually required a I and hour to I packing. SR-30 The 1/2 hours and the peaks quantified using a disc integrator. Experimental Procedure At the beginning of a between the flanges. The run a fresh membrane was placed upper flange was greased with 19 vacuum grease and placed on a sheet of Dupont polypropylene membrane to serve as the cutting pattern. The membrane was cut to the flange. The to wrinkling the membrane size around carefully placed together avoid flanges were then and securely fastened together with the large nut. Twenty to forty grams in the feed cavity. of The the feed mixture were placed amount of feed required was dependent on the number of runs to be made with a membrane. A for fresh membrane composition. Runs was used were made each varying different feed the following three parameters: 1. concentration of the complexing agent 2 . ratio of the para to meta xylene 3. temperature The combinations studied are presented in Table 2. Each set of runs made using a single membrane consisted of a specific mixture of CBr^, p-xylene, and m-xylene, separated Table 2. Various Mole % CBr4 Added to Complex with p-Xylene in the Para and Meta Xylene Feed Mixture. !TEMPERATURE 0C RATIO OF D / m 10/90 30/70 50/50 70/30 90/10 -20 0,10,24 0,10,24 0,10,24 0,10,24 0,10,24 5 0,10,24 0,10,24 0,10,24 0,10,24 0,10,24 22 0,10,24 0,10,24 0,10,24 0,10,24 0,10,24 50 0,10,24 0,10,24 0,10,24 0,10,24 0,10,24 60 10,24 10,24 10,24 10,24 10,24 20 at the various temperatures 220C r 5 0 0C, and 60°C. to significantly the Therefore where there was some approximately -20°C, S 0C r The warmer temperatures did not seem improve CBr^ was present. of CBr^ separation the obtained when no B O 0C runs were only made present. Under these conditions the CBr^ was completely soluble in the feed mixtures. Prior to each run, the cold finger pyrex condenser used to collect the product was cleaned, dried and weighed. dry weight was recorded to use in determining the amount of product collected. After the liquid feed was allowed to system pressure. product with The run the time collected. examination. was assembled, the reach a steady state temperature and the vacuum pump was started. recorded along The At this point the time was feed temperature, and barometric varied This depending on the amount of was determined by visual The maximum run time was during the -200C runs and was about 48 hours. At B O 0C, the run time was usually only about I hour. After a sufficient amount the cold finger disconnected. then warmed to Pyrex The room Pyrex of product was collected in condenser, condenser temperature and the with vacuum line was the product was weighed. temperature, and product weight were recorded. The time, 21 RESULTS AMD DISCUSSION Figure 5 illustrates temperature obtained, varying percent from p-xylene with no CBr^ added. in terms of an x y separation the pervaporation separation at across Figure vs the a factors (a) vs polypropylene membrane 6 presents the same results but diagram, or the % p-xylene in the product vs the % p-xylene in the feed with no CBr4 added. The separation factors agent was added can be obtained when the complexing seen plotted against temperature Figures 7 through 11. Figures effect of adding CBr^ in terms 12 in through 16 present the of % p-xylene in the product vs % p-xylene in the feed. Figure 5 illustrates how the selectivity of the process changed depending on temperature para and meta xylene feed xylene in the feed mixture, selective for p-xylene and mixture. at the all separation factors were greater concentrated in the for p-xylene. product For the the separation process the warmer temperatures. the % p-xylene in the With 10% and 30% p- separation temperatures. than and process was When the 1 .0 , the p-xylene was the process was selective 50%, 70%, and 90% p-xylene mixtures, was selective With 50% for p-xylene only at and 70% p-xylene in the feed mixture, when the temperature was lowered to -200C, the I, OO 0.80 0.60 SEPARATION FACTOR 0.00 0.20 0.40 ((X) 1.20 1.40 22 TEMPERATURE (bC) Figure 5. Separation factors vs temperature at varying percent p-xylene in the para and meta xylene mixture across a polypropylene membrane with no CBr added. 4 23 o % P-XYLENE IN PRODUC O 22 0. 40 0. 60 C % P-XYLENE IN FEED Figure 6 . Percent p-xylene in product as a function of % p-xylene in feed at varying temperatures with no CBr4 added. 24 1.40 o CO m-xylene selective I. OO 0. 60 0.80 p-xylene selective 24% CSr4 .40 (CX) S E P A R A T I O N FACTOR 1.20 0% CBr4 - 40.00 - 20.00 0 . 00 TE M P E R A T U R E 20 . 00 4 0 . 00 60 . 00 (eC) Figure 7. Separation factor vs temperature for 10% p-xylene and varying % CBr^ additions. 25 o tJ- I p-xylene Oa I z «4- w m-xylene selective 10% C B r 4 0.60 0.80 1.00 ■^ 24% CBr4 .20 0.40 S E P A R A T I O N FACTOR (CX) 0% CBr4 -40.00 0 . 00 2 0 . 00 40. 00 TEMPERATURE Figure 8. Separation factor vs temperature for 30% p-xylene and varying % CBr4 additions. 26 o 0% CBr4 0.80 1.00 p-xylene selective m-xylene selective 10% C B r 4 ((X) 0.60 SEP A R A T I O N FACTOR 1.20 CO .40 24% CBr4 - 20.00 0 . 00 20 . 00 TEMPERATURE 40. 00 60. 00 80. 00 (0C) Figure 9. Separation factor vs temperature for 50% p-xylene and varying % CBr^ additions. 27 o 10% C B r 4 0.80 m-xylene selective 0.60 CBr 4 (CX) .20 0.40 S E P A R A T I O N FACTOR 1.00 CBr 4 p-xylene selective -40.00 - 20.00 0.00 20.00 40. 00 60. 00 TE M P E R A T U R E Figure 10. Separation factor vs temperature for 70% p-xylene and varying % CBr^ additions. 28 o CM p-xylene selective 1 0 % CB r 4 0.40 0.60 (CX) m-xylene selective CBr 4 .00 0.20 S E P A R A T I O N FACTOR 0.80 CBr 4 -40.00 - 20.00 0. 00 2JQ. 00 40. 00 60. 00 TEMPER A T U R E Figure 11. Separation factor vs temperature for 90% p-xylene and varying % CBr4 additions. 29 O C CS r 4 0 . 80 P-XYLENE IN FEED Figure 12. Percent p-xylene in product as a function of % p-xylene in feed with 10 and 24 mole % CBr^ added at 60°C. 30 O O 0% CSr4 CS r 4 P-XYLENE IN FEED Figure 13. Percent p-xylene in product as a function of % p-xylene in feed with 0 and 24 mole % CBr added at 500C. 4 31 % P-XYLENE IN PRODU O O 0 % CS r 4 24% CSr4 % P-XYLENE IN FEED Figure 14. Percent p-xylene in product as a function of % p-xylene in feed with 0 and 24 mole % CBr added at 220C. 4 32 O O O o'" 0 % C B r4 2 4 % CB r4 'o. oo o. 40 X 0. 60 C I. oo % P-XYLENE IN FEED Figure 15. Percent p-xylene in product as a function of % p-xylene in feed with 0 and 24 mole % CBr added at 5 0C. 4 33 % P-XYLEME IN PRODUC C O 0% C B r 4 24% CSr4 0.40 0. SO C P-XYLENE IN FEED Figure 16. Percent p-xylene in product as a function of % P-xylene in feed with 0 and 24 mole % CBr added at -20°C. 4 34 selectivity of the xylene was separation concentrated factors are between in zero selective for m-xylene. process the and xylene, the selectivity of product. 1.0 For changed a when feed so that xn- The separation the process was mixture with 90% p- the process was for m-xylene at Figure 6 presents the same results as in Figure 5, but both 5 0C and -20°C. on an x vs y diagram which product as a function of the shows % Points % p-xylene in the p-xylene in the feed. diagram conveniently illustrates of the process. the the This degree of selectivity on the diagonal represent the cases of no separation, where there is the same amount of p-xylene in the feed as in the product. As the degree of separation, or the selectivity of the process, increases, the difference in the concentration of p-xylene the product becomes greater. in the feed from that in This is shown by the points on the x vs y diagram which are farther away from the diagonal. Points above the diagonal because there is a higher product than in the feed. indicate selectivity for p-xylene concentration of p-xylene in the Points located below the diagonal represent process selectivity for m-xylene, or less p-xylene in the product than in greatest change in -200C and where the the feed. selectivity feed Figure 6 shows that the occurs mixtures at a temperature of have a concentration of 35 p-xylene of 50% and higher. Under these conditions the process is selective for m-xylene. The separation factors obtained at various temperatures with varying percent additions of CBr4 are shown on separate graphs for each mixture. different The experimental concentration of p-xylene Under the percent conditions results was with p-xylene 10% no are CBr4 in when the feed the feed shown on Figure 7. and 10 mole % CBr4 additions, the selectivity of the at all temperatures. When 24 mole feed mixture and the temperature was -200C, the selectivity of the process was for process was for p-xylene % CBr4 was added to the m-xylene. warmer temperatures resulted in Experimental runs at the the process being selective for p-xylene. Figure 8 illustrates the results xylene in the feed selectivity of the mixture. When process addition of 10 mole % CBr4 , when there was 30% p- was the no CBr4 was added, the for With the selectivity of the process was for p-xylene at temperatures of -200C the selectivity changed p-xylene. so 5°C and warmer. But at the process was selective for m-xylene. When 24 mole % CBr4 was selective for p-xylene at 22°C at both 5 0C and -200C was evaluate the reproducibility which had 30% p-xylene in the added r the and warmer. for of m-xylene. the feed process was The selectivity In order to data, the experiments mixture with 24 mole % 36 CBr^ added were duplicated. The results are shown on Figure 8 . Figure 9 presents the experimental xylene feed composition was 50% separation process when no CBr^ m-xylene at -200C. At was still selective for p-xylene. the warmer temperatures the process p-xylene to the feed mixture, the 10 mole % CBr4 xylene. when there was no CBr4 With 10 mole % CBr4 added process was selective for m-xylene For the remaining experimental results at addition, A 24 mole temperatures This time the was added was selective for added to the xylene feed mixture. at 5 0C and -200C. results when the of % the CBr4 -200C, process was selective for p- addition to the feed mixture at 5 0C, separation process being and selective 60 0C for resulted m-xylene. in the At 22°C and SO0C the process was selective for p-xylene. Figure 10 depicts the feed mixture the experimental results found when contained 70% p-xylene. separation process was selective the amount of CBr mole % CBr4 added. added, When 24 mole % selectivity of GBr4 the process was the for m-xylene regardless of For the cases with no CBri and 10 where warmer, the separation At -200C the temperatures was selective for p-xylene. added process were 5 0C and to the xylene mixture, the was for m-xylene at all temperatures. Figure 11 illustrates the experimental results obtained with 90% p-xylene in the feed mixture. The separation 37 process with this feed mixture amount of CBr^ added, was CBr4 had been added, the xylene at 5°C, but at selective xylene at all When no the warmer temperatures, the process With the feed mixture, the process 5 0 °C. xylene at 60°C. for m-xylene. process was still selective for m- was selective for p-xylene. 5 0C, 22°C, and at -20°C, regardless of the 10 mole % CBr^ added to was selective for p-xylene at But the selectivity changed to m- The separation process was selective for mtemperatures when 24 mole % CBr^ had been added to the feed mixture. Figure 12 shows the temperature of 600C in experimental results produced at a terms of results when 10 mole % and 24 feed mixture of varying The for m-xylene when mole mixture containing xylene. a x mole % CBr vs y diagram. 4 concentrations xylene are compared. 24 an were added to the of p-xylene and m- separation process was selective % CBr^ concentration In these cases. The Figure was of added to the feed 50% or greater p- 12 shows the % p-xylene in the product was less than the % p-xylene in the feed. Figures 13 through 16 when 24 mole % CBr^ when no CBr^ was when the was compare the experimental results added present. separation to the feed, with the runs Figure 13 presents the results process was operated selectivity was for m-xylene at or greater experimental p-xylene. Figure 14 were collected The at a at 50°C. The feed concentrations of 70% temperature results shown in of 22°C. The 38 selectivity of the process was p-xylene in the feed for mixture. . experimental results obtained when operated temperature at a process was selective for Figure from 15 shows the the separation process of 5 0C. The separation m-xylene at all concentrations of p-xylene in the feed except 10%. temperature of -20°C, m-xylene at 70% and 90% Figure 16 shows that at a all experimental runs were selective for m-xylene regardless of the concentration of p-xylene in the feed mixture. Discussion of Results Background separability data for para and meta xylene across a polypropylene membrane were produced at the desired temperatures. This background which the effect of could be separation factors But at the higher As These the p-xylene with the CBr^ are the Figure greater is concentrations are the 5 shows, than membrane temperatures the pervaporation xylene. served as a basis from complexing compared. surprising since data 1.0. of This the is not selective for p-xylene. of p-xylene and the lower process points most below is selective for mthe 1.0 separation factor line. The pervaporation process is selective for m-xylene at high concentrations of p-xylene and low temperatures because of the crystallization that occurs membrane. Pure p-xylene freezes on the feed side of the at 13.30C, m-xylene is added, the freezing point is depressed. but when Table 3 39 presents the initial crystallization temperatures for the binary mixtures of p-xylene and m-xylene (2 1 ). Table 3. Initial Crystallization Temperatures for Binary Mixtures of p-Xylene and m-Xylene. % P-Xvlene Temp 0C 100 90 70 50 30 13 10 0 13.3 10 - I -13 -29 -52.8 -52 -47.8 For any concentration of temperature is temperature, lowered pure para and meta xylene, as the the initial crystallization below p-xylene crystallizes minimum temperature of -52.B 0C the binary eutectic of the is mixture xylene and m-xylene crystallize the solid phase at the reached. eutectic out until the At that point is reached and both p- out. The concentration of is 13% p-xylene and 87% m- xylene. Crystallization affected the separation of the pervaporation process at feed concentrations of 50% p-xylene and higher when the temperature was xylene, the p-xylene does begin -290C. experiments Since the temperature of -230C, the were not affected by the not 30% below were 5 0C. At 30% p- to crystallize until run at a minimum and lower p-xylene mixtures crystallization of p-xylene. This 40 can be seen on Figure 6, as temperature the pervaporation even process xylene at 30% and lower p-xylene and 70% p-xylene feed and was selective for p- mixtures. mixtures , crystallize at about -13°C for runs at the lowest the - I 0C In both the 50% p-xylene begins to respectively. -200C runs had crystallization, but not So the the S 0C run. For a 90% p-xylene mixture, p-xylene will begin to crystallize out at IO0C, therefore both the 5 0C and -200C runs had crystallization. As can be seen, in every run where crystallization was present, the selectivity of the xylene. Since the p-xylene cannot permeate through the So although the that is in crystalline form, it membrane, but the m-xylene can. membrane conditions are such membrane process was for m- is selective crystallization for p-xylene when is present, the membrane process is selective for m-xylene. P-Xylene, m-Xvlene, and CBr 4-System ----- --------C---The complexing agent, CB r ^ , was mole % and 24 mole mixtures. It was % to the various para and meta xylene expected that an equimolal C B r ^ • p-xylene complex would form as shown by Egan mole % CBr^ runs, the mixture xylene to utilize all the at the 10/90 ratio added in amounts of 10 other run at 10 mole % (22). In the 10 in which there was enough p- CBr^ p-xylene et al. as an equimolal complex was to m-xylene. Therefore, every CBr^ had a p-xylene concentration in 41 excess of that required to the 24 mole % CBr^ runs r complex For an equimolal ratio of p-xylene to CBr^ existed where there was the feed mixture. with all the CBr^. There a 30/70 para to meta ratio in was excess p-xylene present for para to meta ratios greater than this. The results was selective which for m-xylene through 16, and can be m-xylene, and CBr^ indicate the pervaporation process are presented interpreted freezing in Figures 7 in terms of a p-xylene, point diagram, and selective complex formation between the CBr4 and p-xylene. The behavior of para and meta xylene in the presence of CCl4 was studied conveniently by Egan represented by diagram (shown in Figure divided into four areas et al. the 17a). to (23). The ternary data was freezing point The triangular diagram is indicate the composition of the solid phase that crystallizes out first when a solution of a given composition is cooled. In the same manner, has been constructed system. a for ternary freezing point diagram the This diagram is shown the triangle represents a p-xylene, m-xylene, and CBr4 in Figure 17b. binary mixture. Each side of Information on the initial crystallization temperatures of the binary mixtures were taken from plots located in the article by Egan et al. An equimolal complex of and the eutectics (also shown in Figures I and 2) (24). p-xylene and CBr4 is shown on the 42 P-XYLENE F.P .13.3 0C MOLE BASIS dashed lines are isotherms solid lines are eutectics p-xylene \\F.P CCl •p-xylene m-xylene M-XYLENE F.P. F.P. -22.S 0C P-XYLENE F.P. 13.3 0C 12.B 0C p-xylene m-xylene M-XYLENE F.P. 'Br -m-xylene Figure 17. Freezing point diagrams for mixtures of p-xylene, m-xylene, and CCl , and p-xylene, m-xylene, and CBr . 4 43 right side of the triangle and has a freezing point of 53.3°C. CBri^ also forms a conditions. Therefore complex the for p-xylene, m-xylene, labled areas. Each ternary and area with m-xylene under certain freezing point diagram CBr4 , is indicates divided into five the composition of the solid phase that crystallizes out first when a solution of a given composition is separating the areas assumption in cooled. are Figure quaternary (point A) The the 17b binary concerns between the five solid eutectics. the lines The main location of the CBr4 , the complexes r and the xylenes. The eutectic between para and meta xylene is shown as a horizontal dashed line following the -52,S 0C isotherm. The quaternary will be located somewhere along this p-xylene and m-xylene eutectic, at the point of intersection of the other eutectics. The separation factor resulting from the experimental run having a feed mixture of 30/70 para to meta xylene with 10 mole temperature of -200C solid formation % CBr4 was addition below occurring which 1.0. (point B) at a This indicated some effectively changed the separation factor making the process selective for m-xylene. If the solid forming CBr4 • p-xylene complex, about "-34.4°C (note below point B ) . the were the temperature -34.40C Therefore p-xylene the instead would of the have to be. isotherm located slightly solid forming must be the 44 CBr^' p-xylene complex -20°C. So, the since the temperature is only at p-xylene — CBr4 • p-xylene eutectic must be located to the left of point B. Determination of the exact location for the is beyond research. The quaternary phase diagram is the scope of this nevertheless helpful in interpreting the experimental results. The quaternary formed is located at the intersection of the lines representing p-xylene — CBr4 • p-xylene eutectic, CBr4 ' p-xylene — CBr4 - m-xylene m-xylene eutectic, (point A). the and the eutectic, CBr4 - m-xylene — m-xylene — p-xylene eutectic A ternary is also shown at point C, intersecting CBr4 - p-xylene - CBr4 eutectic, CBr4 - CBr4 - m-xylene eutectic, and CBr4 - m-xylene — CBr4 - p-xylene eutectic. The isotherms in lines. Figure The isotherms in in Figure 17a. This 17 Figure is 17b are more vertical than due to freezing points of CBr4 and CCl4 . in Figure 17b go from triangle since there right side of the the are left no difference in the the bottom side of the negative temperatures on the Although xylene, and CBr4 ternary freezing approximation, it proves helpful on the The negative temperatures to triangle. effects of crystallization are represented by dashed the p-xylene, m- point diagram is only an in the discussion of the the pervaporation separation of para and meta xylene in the presence of CBr4 . The results of the 10/90, to meta xylene mixtures at 30/70, and 50/50 ratio para 22 0C and above were all similar. 45 The change in separation factors, a separation factor less than of CBr^ added except at 50% 6 0 0C. At concentrations feed, no solid if any, did not result in 1.0 regardless of the amount p-xylene with 24 mole % CBr4 at of 50% and CBr4 • p-xylene less p-xylene in the complex formed at room temperature of 220C. It was desired to know what composition of p-xylene was required in the feed mixture complex at room temperature conducted to find formed. to form a solid CBr4 • p-xylene at what of 22 0C. ratio of p-xylene the complex The analyses were conducted with 24 mole % CBr4 for varying ratios of para to meta xylene. % CBr4 in liquid as a function concentration at 22°C and decrease GC analyses were in CBr4 in liquid concentration of CBr4 was 24 mole xylene mixture complexes with CBr4 that is detected by the CBr4 detected in the of the feed p-xylene illustrates graphically where the the solid complex formation is Figure 18 shows the occurs. %. the initial When the CBr4 in the p-xylene, the amount of GC is reduced. indicated liquid The by phase. Therefore the a decrease in the The CBr4 - p-xylene complex first forms when there is 24 mole % CBr4 present and when the ratio of para to meta xylene is 60/40. remains in solution at concentrations of p-xylene less than 50% of the para and meta xylene mixture. in the liquid decreases as to the formation of the the The CBr4 The amount of CBr4 % p-xylene is increased due CBr4 - p-xylene complex. There is 20.00 10.00 .00 MOLE % CBr4 30.00 46 ° 0 . 00 20. 00 40. 00 60. 00 80. 00 % P-XYLENE IN XYLENE MIXTURE ioo. oo Figure 18. Mole % CBr present in liquid phase as a function or p-xylene concentration in the para and meta xylene mixture at 22 0C with an initial concentration of CBr^ at 24 mole %. 47 also a corresponding decrease in the p-xylene concentration in the remaining liquid. This explains how even though the membrane is selective f or p-xylene, when the CBr4 * p-xylene complex is formed, for m-xylene. the pervaporation Since some of complex, it cannot permeate m-xylene continues - to greater concentration results in a very good the p-xylene is tied up in the through permeate of process is selective the membrane. and m-xylene so in the the But the result is a product. This separation of m-xylene. from p-xylene by the pervaporation process. For the 10/90 runs, a enough to cause any temperature xylene of 5 0C was not cold crystallization. Since the p- xylene concentration was low, no complex was formed. -20°C there was a significant factor with the addition of 24 the separation factor can phase diagram in Figure temperature was low phase that would xylene. be CBr4 -P-Xylene complex.' the separation mole % CBr4 . This change in At the crystallize 10 in explained by referring to the 19. enough, Therefore at change At 24 mole % CBr4 , if the composition out mole 10 % mole of the solid first would be pure in- CBr4 there would be no % CBr4 , the solid phase which would form first is the CBr4 -p-xylene complex. -200C the CBr4 -P-Xylene effectively pervaporation changes process. the But at complex forms separation The in the factor pervaporation So, at feed and for process the is 48 100% P-XYLENE F.P 13.3 0C p-xylene MOLE BASIS dashed lines are isotherms solid lines are eutectics CBr •p-xylene /,10/90 ratio xylene • /rni v h i iy a Tzri I OSc P-R 100% M-XYLENE C CBr "m-xylene F.P. -47.S0C Figure 19. Freezing point diagram for mixtures of p-xylene, m-xylene, and CBr ^ . 100% CBr F.P. 86= n* m-xylene 49 now selective for m-xylene as the m-xylene is concentrated in the product. For the experimental runs meta xylene feed mixture, with solid a complex feed did occur at -200C for both additions. containing Only the feed solid complex formation at 5°C. process became more amounts of CB r ^ . the 10 mole % complex was formed. of complex was greater more CBr4 was added, more the amount of p-xylene available membrane xylene available to permeate m-xylene permeation mole % CBr^ had 24 mole % CBr4 runs, the amount so to permeate through the 24 was an excess of p-xylene for as In the the 10 and 24 mole % CBr for m-xylene with increasing Since there case, formation in the At -20°C, the pervaporation selective CBr4 30/70 ratio para to rate was through less. With less p- the membrane, and the unchanged, the m-xylene ends up being concentrated in the product. For the 50/50 ratio solid complex para formation mixtures with both 10 and to meta xylene mixture runs r occurred at 5 0C 24 % CBr . mole and The more CBr 4 present, the more complex was leads to the m-xylene being formed, the feed. The p-xylene and as before, this from p-xylene. At -20°C, p-xylene would crystallize out in in crystalline form cannot permeate through the membrane so this process to m-xylene. 4 concentrated in the product and a greater separation of m-xylene with no CBr^ present, the -20°C for changes the selectivity of the 50 For both, the 70/30 and 90/10 mixtures, complexing was apparent in the runs with 24 addition of 10 change the added. mole mole % CBr^ to selectivity from the % CBr^ as expected. the The feed mixture did not case where no CBr4 was This indicates the temperature was not low enough to cause any solid complex formation. factors for 0, 1 0 , and 24 this case, there both 10 and 24 is mole solid mole% CBr At -20oC, the separation % CBr4 were very close. complex In formation occurring at additions, and when no CBr 4 is 4 added, pure p-xylene crystallizes out. The results for the 70/30 ratio para to meta run can be seen by referring to the phase diagram in Figure 20. 24 mole % CBr4 , with a mixture for the other 76 Beginning at point A, which is 70/30 ratio (para to meta) xylene mole %, cooling the mixture would crystallize out CBr4 - p-xylene crystallize out until about remaining crystallization xylene — p-xylene would occur (down same would The complex would (B), to approximately remaining xylene mixture This is roughly the -12°C eutectic final temperature of complex. at which point the along point -200C the CBr4 - pC). Mhen the was obtained the contain about 42% p-xylene. product concentration of p-xylene which was obtained experimentally. At 10 mole % CBr4 following E, pure p-xylene would crystallize temperature of -200C about 42 in the liquid. As for the cooling line from D to the out. At the final % p-xylene would again remain case with no CBr4 added, using 51 P-XYLENE F.P. 13.3°C MOLE BASIS dashed lines are isotherms solid lines are eutectics 10% CBr p-xylene 24% CBr 40% p-xylene -23.3 °C 38% p-xylene CBr »p- xylene m-xylene M-XYLENE F.P. Figure 20. Freezing point diagram for mixtures of p-xylene, m-xylene, and CBr4 . 52 th.e phase diagram, a final approximately 36 % would be xylene concentrations p-xylene expected. are concentration of Since these final p- similar to concentrations found experimentally, it the product is evident that the solid complex formation or pure p-xylene crystallization are the predominant separating concentrations and low mechanisms at the high p-xylene temperatures. Both situations decrease the amount of p-xylene in the feed which is free to permeate through the membrane. So, although the membrane itself is selective for p-xylene, under the conditions where the solid complex formation present, xylene. the of p-xylene crystallization are pervaporation The separation of process is selective for m- the xylenes is greatly enhanced over the separation obtained by just pervaporation. Formation of CBr^ * p-xvlene Association in Liquid Phase For the experimental runs 50% and higher selective to change in p-xylene, m-xylene selectivity formation between the the at the cannot CBr^ and melting points are as follows: with a feed concentration of membrane process became more higher be due temperatures. to solid This complex p-xylene since the complex 53 Table 4. Melting Points for the CBr • p-Xylene Complex at High Concentrations of p-Xylene in the Xylene Feed Mixture. % p-Xvlene in Feed mole % CBr 4 50 70 70 90 90 Meltincr Point 0C 24 10 24 10 24 <22 °C <22°C 37 °C <22°C 45 0C At temperatures of 50°C and 60°C, the complex is in the liquid phase for all feed compositions examined. in selectivity must CBr^ and p-xylene be due which The p-xylene in the to an association between the effectively association through the membrane and The change ties up the p-xylene. is unavailable to permeate therefore the process is selective for m-xylene. Carbon Tetrabromide in the Product The products from the 10 mole % CBr^ and 30/70 ratio para to meta xylene mixture were analyzed for CBr^ using an infrared spectrometer (IR). The in all products tested with presence of CBr^ was found IR. The products were then analyzed with a GC and the results are shown in Table 4. Table 5. CBr Present in the product of the 30/70 Ratio (para to meta) Xylene Run with 10 mole % CBr^. [Temperature I pole % CBr^ | -20°C 0.00 | 7°C 3.02 | 22°C 3.11 | 50°C 3.02 | 59°C 2.93 | 54 As can be seen the CBr^ did product of the -200C run indicated by the IR, it not show up on the GC when the was was analyzed. a Although it was very weak peak indicating a very low concentration. Advantacre of Using Pervaporation Process The separations occurring solid complex formation to be as good pervaporation. as If in the feed resulting from and p-xylene crystallization appear these separations anything, without pervaporation across they the would combined with be membrane formation and crystallization both the liquid phase of the when a bit better since the solid decrease the p-xylene in feed, and the pervaporation process would take what p-xylene is available in the liquid phase of the feed and concentrate it in the product. the m-xylene is being concentrated on the feed side of the membrane, but diluted on the product side. greater percentage of m-xylene in the In other wor d s , There would be a liquid phase of the feed than in the product. One advantage of separation by means of xylene crystallization present in the product. using the pervaporation process over just solid complex formation and p- exists in the reduction of CBr^ Without pervaporation, the amount of CBr^ present in the liquid was still about 8 mole % with an excess of p-xylene and no m-xylene (see Figure 18). m-xylene was introduced into the When system, the amount of CBr^ 55 remaining in the liquid increased. The CBr4 present in the product of pervaporation separation regardless of the amount of para or meta xylene present was at most 3.11 mole %. end uses of the separated as to the effect of CBr4 The xylenes would have to be examined as an impurity. There would also be some cost involved from the loss of the CBr4 . Effect of Complexing- Aaent on Flux The average flux, as a function of temperature for each mole % CBr4 is shown Figure 21 that the in flux Figure 21. decreases amount of the CBr4 -p-xylene It is evident from with an increase in the complex. This is probably due to the fact the membrane is selective for p-xylene, and thus p-xylene permeates faster than is complexed with the CBr4 , m-xylene. it xylene present, which results in reduces When the p-xylene the amount of p- an overall decrease in the total flux because of the lower permeation rate of m-xylene. Therefore the more lower the flux. 10, and 24 mole % CBr4 The added, flux CBr4 the more complex and the for the individual runs with O , added, along with the average flux for the runs at each temperature are located in Table 6 . 56 o o (kg/m2hr) 0% CSr4 1 0 % CS r 4 FLUX 24% CSr4 - 20 . 00 0.00 20 . 00 TEMPERATURE 40. 00 60. 00 (bC) Figure 2 1 . Average flux for pervaporation process with 0, 10, and 24 mole % CBr added. 4 80. 00 57 Table 6. Flux For the Runs at 0, 10, and 24 Mole % CBr^ a. Flux in kg/m nr for varying temperatures and concentrations with 0% CBr . 4 CONC. ratio p/m 10/90 30/70 50/50 70/30 90/10 TEMP °C -20 5 22 50 0.023 0.111 0.121 1.623 0.034 0.048 0.310 1.921 0.021 0.158 0.097 1.302 0.014 0.146 0.169 1.536 0.022 0.078 0.281 2.280 Flux in kg/m2hr for varying temperatures and concentrations with 10 mole % CBr . OTVTr* 4 CONC. ratio p/m 10/90 30/70 50/50 70/30 90/10 TEMP 0C AVE 0.023 0.110 0.200 1.730 b. -20 5 22 50 60 0.010 0.055 0.076 1.106 1.911 0.014 0.080 0.215 1.129 2.019 0.007 0.064 0.103 1.358 1.922 0.032 0.088 0.110 1.256 2.260 0.018 0.100 0.131 1.106 1.896 Flux in kg/m2hr for varying temperatures and concentrations with 24 mole % CBr . CONC. ratio p/m 10/90 50/50 70/30 90/10 30/70 TEMP 0C AVE 0.020 0.077 0.130 1.190 2.000 c. -20 5 22 50 60 0.002 0.040 0.041 0.650 1.313 The flux did 0.008 0.025 0.025 1.060 1.527 0.015 0.018 0.080 0.743 1.580 not appear during separations where flux was below 0.2 to 0.040 0.072 0.108 1.627 2.020 be 50°C. for most At 50°C 0.014 0.030 0.080 1.110 1.760 appreciably reduced crystallization kg/m2hr temperature was below 0.003 0.019 0.136 1.493 2.384 AVE was present. The of the runs when the the flux increased 58 significantly, ranging from 1.3 JcgZm2Iar to 2.3 JcgZm2Iir This is about a 10 fold increase over the 2 0 0C flux. 59 CONCLUSIONS 1. CBr^ forms a solid molecular complex with, the p-xylene and therefore decreases the amount of P-xylene in the pervaporation product at high concentrations of p-xylene and low temperatures. 2. In general, the addition of 24 mole % CBr^ changed the separability of the pervaporation process so it was selective for m-xylene at low temperatures for all concentrations of p-xylene, but the 10 mole % CBr^ addition only affected the separability at low temperatures for higher concentrations of p-xylene. 3. The degree of separation varies with temperature, the greatest separation in the presence of CBr^ occurring at -20°C where solid complex formation was present. 4. At 220C, 24 mole % CBr^ complexes with p-xylene when the xylene mixture has at least 60 wt % p-xylene (and 40 wt % m-xylene). 60 CONCLUSIONS— Continued 5. At temperatures of 500C and S O 0C r where the solid complex has melted and there is above 50% p-xylene in the xylene feed mixture, an apparent association is present between the CBr4 and the p-xylene which ties up the p-xylene in the feed and changes the process selectivity to m-xylene. 61 SUGGESTIONS FOR FUTTTRFl PRREARCH I* Different coitiplexing agents should, be experimentally evaluated, to find one which complexes with m-xylene. This would be advantageous since the complexing agent would then be working with the membrane's selective nature instead of against it, as with the CBr . 2. Determine the behavior of the molecules in terms of a phase diagram so the conditions where the CB r ^ -p-xylene complex forms can be more accurately predicted for specific compositions and temperatures. 3. Continue the current experimental research at temperatures above 6 0 0C to see if the trend towards greater m-xylene selectivity continues. Examine the association formed between the CBr 4 and the p-xylene at these higher temperatures. 62 LITERATURE CITED 1. McCandless, F. P . , and D o w n s , W . B . , "Separation of C Aromatic Isomers by Pervaporation Through Commercial Polymer Films," Journal of Membrane Science, V o l . 30, pp. 111-116 (1987). 2. Y e h , A., "A Study of the Reversing of Relative Volatilities by Extractive Distillation," Thesis, Montana State University, Bozeman, MT (1980). 3. Downs, W.B., "Temperature Effects on the Separation of Isomeric Xylenes Using the . Pervaporation Process," Thesis, Montana State University, Bozeman, MT (1985). 4. Morbidelli, M., Santacesaria, E., Giuseppe, S., and Carra, S., "Separation of Xylenes on Y Zeolites in the Vapor Phase. 2. Breakthrough and Pulse Curves and Their Interpretation," Ind. Eng. C h e m . Process D e s . D e v . , V o l . 24, pg 83 (1985). 5. Morbidelli, M., Giuseppe, S., and Carra, S., "Comparison of Adsorption Separation Processes in the Liquid and Vapor Pha s e . Application, to the Xylene Isomer Mixture," Ind. Eng. Chem. Fundam., V o l . 25, pp. 89-95 (1986). 6. Morbidelli, M., Santacesaria, E., Giuseppe, S., and Carra, S., "Separation of Xylenes on Y Zeolites in the Vapor Phase. 2. Breakthrough and Pulse Curves and Their Interpretation," pp. 83-88. 7. Morbidelli, M., Giuseppe, S., and Carra, S., "Comparison of Adsorption Separation Processes in the Liquid and Vapor Phase. Application ot the Xylene Isomer Mixture," I nd. Eng. C h e m . Fundam., V o l . 25,pp. 89-95 (1986) . 8. Morbidelli, M., Santacesaria, E., Giuseppe, S., and Dar r a , S., "Separation of Xylenes on Y Seolites in the Vapor Phase. 2. Breakthrough and Pulse Curves and Their Interpretation," pp. 83-88. I LITERATURE CITED— Continued Binning, R.C., Lee, R.J., Jennings, J.F., and Martin, E.C., "Separation of Liquid Mixtures by Permeation," Industrial Engineering and Chemistry, Vol 53, No I, pg 45 (1961). Sikona, J.G., and McCandless, F.P., "Separation of Isomeric Xylenes by Permeation Through Modified Plastic Films," Journal of Membrane Science, V o l . 4, p p . 229-241 (1978). Michaels, A.S., Baddour, R.F., Bixler, H.J., and Choo, C. Y . , "Conditioned Polyethylene as Permselective Membrane. Separation of Isomeric Xylenes.," Industrial and Engineering Chemistry Process Design and Development, Vol I, No I, pg 14 (1962). McCandless, F.P., and Downs, W . B . , pg 112. Torrey, S., "Membrane and Ultrafiltration Tech­ nology Developments Since 1981," Chemical Tech­ nology Review No. 226, Noyes Data Corporation, pp. 425-435 (1984). Sikona, J.G., and McCandless, F.P., "Separation of Isomeric Xylenes by Permeation Through Modified Plastic Films," Journal of Membrane Science, V o l . 4, pp. 229-241 (1978). Torrey, S., "Membrane and Ultrafiltration Technology Developments Since 1981." p 438 Lonsdale, H. K . , MiTstead, C.E., Cross, B.P., and Graver, F.M., "Study of Rejection of Various Solutes by Reverse Osmosis Membranes," Technical Report No. 447, Prepared by Gulf General Atomic, Inc., for the Office of Saline Water, Available Through NTIS (PB 203828), Springfield Virginia, March (1969). Strathmann, H . , and K o c k , K . , "Selective Removal of Heavy Metal Ions from Aqueous Solutions by Diafiltration of Macromolecular Complexes," in N.N. Li (Ed), Recent Developments in Separation Science, V o l . IV, CRC Press, Boca Raton, Florida, pp. 29-38, (1978). 64 H CO LITERATURE CITED— Continued E g a n r C.J . r and Luthyr R . V . r "Separation of Xylenesr" Industrial and Engineering Chemistry, V o l . 47, No. 2, p p . 250-253 (1955) 19. Ibid, p. 252. 20. Ibid, p. 252. 21. Ibid, p. 251. 22. Ibid, p. 252. 23. Ibid, p. 251. 24. E g a n , C .J ., and Luthy, R . V . , "Separation of Xylenes," p 250. v 65 APPENDIX CROSS PLOTS FOR FIGURES 13 THROUGH 17 66 ALPHA o - 20.00 0.00 20 . 00 40. 00 60. 00 TEMPERATURE Figure 22. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 10% p-xylene in the xylene mixture with no CBr added. 4 I . 16 , .08 I . 10 I . 12 1.14 AL P H A 1.18 1.20 67 " - 4 0 . 00 -20.00 0.00 20.00 TEMPERATURE 40.00 60.00 C Figure 23. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 30% p-xylene in the xylene mixture with no CBr added. 4 68 CO ALPHA CM - 20.00 20.00 40.00 TE M P E R A T U R E 60. 00 80. 00 C xi Figure 24. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 50% p-xylene in the xylene mixture with no CBr^ add e d . 69 ALPH O 0.00 20.00 TEMPERATURE 40. 00 60. 00 C Figure 25. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 70% p-xylene in the xylene mixture with no CBr a d ded. 4 70 ALPHA O <N - 20.00 0.00 20.00 40. 00 60. 00 TEMPERATURE Figure 26. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 90% p-xylene in the xylene mixture with no CBr added. 4 1.20 71 + 0.80 + 0.00 0.20 0. 40 0.60 ALPHA 1.00 + - 20.00 0 . 00 20.00 40.00 TEMPERATURE 60.00 80.00 C Figure 27. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 10% p-xylene in the xylene mixture with 10 mole % CBr added. 4 72 O ALPHA CM C CO Ol o~ CO CO C C CO G~ CM - 4 0 . OO - 20.00 0.00 20.00 TEMPERATURE 40. 00 60. 00 C Figure 28. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 30% p-xylene in the xylene mixture with 10 mole % CBr 4 added. 73 o AL PH A CM - 20.00 0.00 20.00 40.00 TEMPERATURE C 60. 00 80. 00 Figure 29. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 50% p-xylene in the xylene mixture with 10 mole % CBr added. 4 74 ALPHA C TEMPERATURE Figure 30. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 70% p-xylene in the xylene mixture with 10 mole % CBr added. 4 75 O 0.80 0.20 0.40 0.60 ALPHA 1.00 CM O O ° - 2 0 . 00 0.00 20.00 40.00 60.00 80.00 T E M P E RATURE C Figure 31. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 90% p-xylene in the xylene mixture with 10 mole % CBr added. 4 76 o ALPHA n - 40.00 0.00 20.00 J 60 . 00 TEMPERATURE C Figure 32. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 10% p-xylene in the xylene mixture with 24 mole % CBri added. 4 77 ALPH C TEMPERATURE C Figure 33. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 30% p-xylene in the xylene mixture with 24 mole % CBr added. 4 78 O ALPHA CM - 20.00 20.00 40.00 TEMPERATURE C Figure 34. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 50% p-xylene in the xylene mixture with 24 mole % CBr 4 added. 0.64 .32 0.40 0.48 0.56 ALPHA 0.72 0.80 0.88 79 _________ I ° - 4 0 . 00 -20.00 __________ __________ __________ 0.00 20.00 TEMPERATURE 40.00 60.00 C Figure 35. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 70% p-xylene in the xylene mixture with 24 mole % CBr added. 80 S ALPHA d -20.CO 0.00 20.00 40. OO temperature C 60. oo Figure 36. Separation factors (a) produced as a function of temperature from the pervaporation separation of a feed mixture containing 90% p-xylene in the xylene mixture with 24 mole % CBr added. 4 ITnDlHIES 1762 luu I