Energy Week 2
advertisement
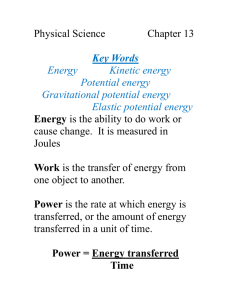
Energy Week 2 October 12-16 October th 12 2015 page 67 DO: I will be able to breakdown the relationships as well as unique characteristics the forms and types of energy as well as heat transfer in everyday instances. EQ: Explain the characteristics of Potential and Kinetic Energy. WARM UP: 24. A molecule of hydrogen peroxide is shown. Which of the following shows the correct chemical formula for this molecule? Use the diagram below to answer question. HH O A. HO C. HO2 O H B. H2O2 D. H2O GOALS Make goals for this energy unit. What did you do well to be successful in the Matter unit? What could you improve on for the energy unit? Explain the transformations: October th 13 2015 page 69 DO: I will be able to breakdown the relationships as well as unique characteristics the forms and types of energy as well as heat transfer in everyday instances. EQ: 1.Describe how all energy comes from the SUN; give examples to clarify your explanation. 2. Explain the relationship between the Law of Conservation of Matter and the Law of Conservation of Energy. Warm UP Transformations • The purpose of a nuclear power plant is to transform nuclear energy into electricity. This is done is steps starting with a nuclear reaction which generates heat to produce steam. The steam in turn is used to turn a generator which finally produces electricity. If you were to create an energy transformation which type of energy would come first? Second? Third? Fourth? REVIEW of TRANSFORMATIONS Closure • QUIZ. Complete the Quiz to show what you KNOW about energy. October th 14 2015 page 71 DO: I will be able to breakdown the relationships as well as unique characteristics the forms and types of energy as well as heat transfer in everyday instances. EQ: 1.Describe how all energy comes from the SUN; give examples to clarify your explanation. 2. Explain the relationship between the Law of Conservation of Matter and the Law of Conservation of Energy. Warm UP 36. The number placed below and to the right of an element’s symbol in a chemical formula is called a A. subscript B. coefficient C. reactant D. product Lets Review… Chemical Reaction or a Chemical Formula Coefficients Reactants Products Subscripts *Cannot be changed when balancing equations My thoughts page 70 •Explain how the selection connects to what we are learning. EX: ? nuclear to thermal to mechanical to electrical BEFORE THE VIDEO IN THE VIDEO •Identify as many types of energy as possible briefly explain each one. •Label as many transformations as possible. My thoughts page 70 •Explain how the selection connects to what we are learning. EX: nuclear to thermal to mechanical to electrical BEFORE THE VIDEO 1. radiant to light AND thermal energy; 2. mechanical to sound energy IN THE VIDEO •Identify as many types of energy as possible briefly explain each one. •Label as many transformations as possible. DANGER Why is this dangerous? How does the types of energy produced make this a hazard? Closure 1 .What types of Kinetic and Potential energy are present in this picture? 2. Compare and Contrast the Potential and Kinetic Energy that you observe in this picture. Explain how Potential and Kinetic energies work together, while still maintaining an inverse relationship. 3. Specifically explain of thermal exchanges are present as a candle burns? 4. How many energy transformations are occurring? 5. Are the changes in the candle as it burns chemical or physical? 6. Using the candle pictured as a reference, explain how the Law of Conservation of Matter and Energy work together in all transformations. Closure Breakdown: Explain the types of energy transformations at each number. 6._______ 7. _______ 5. 1. _______ 2. _______ 3. _______ 4. _______ October th 15 2015 page 73 DO: I will be able to explain connections within the overall concept of Energy and then explain the relationships as well as unique characteristics the forms and types of energy. EQ: How does energy connect with concepts from within our study of matter? How does energy connect with concepts in biology specifically ecological systems? How does energy connect with concepts within astronomy? Warm UP If the reactants contain 12 carbon atoms and 4 oxygen atoms, what must be true of the products? A. B. C. D. The product must be a molecule made of 16 atoms The products must contain 4 carbon atoms and 12 oxygen atoms. The products must consist of carbon to oxygen atoms in a 1:1 ratio. The products must contain 12 carbon atoms and 4 oxygen atoms. Review: •Explain the common scientific misconceptions associated with each system? 1 2 3 The flame is conducting the heat to the wick. This shows kinetic energy happening With the plant using photosynthesis. The wind is electrical energy. Misconception Breakdown: Explain the types of energy transformations at each number. Radiation- Light Thermal-Convection Gravitational Potential Thermal- Conduction Thermal Heat- Radiation transfer of energy Chemical Reaction Thermal Heat- Conduction transfer of energy Gravitational Potential, Conduction, Physical Phase Change. Chemical Potential 12 Dashing-KM; Snow- T: endothermic, GP; Horse- KM, KT, CP, KS,KF, GP; Sleigh-GP, CP, & KF. KINETIC POTENTIAL Mechanical = KM Gravitational = GP Radiant = KR Elastic= EP Sound= KS Nuclear = NP Friction= KF Chemical = CP Electricity = KE Thermal = T endothermic or exothermic Homework 1. Finish Breaking down Jingle Bells verse. 2.Complete the Back of that page using MRS.FET and GENC. Exit Ticket: Explain the PE and KE of the ball at 1,2,3, and 4. Write a sentence Summarizing the relationship of Potential and Kinetic energy. What is the relationship between Potential and Kinetic energy? 1 2 3 4 October th 16 2015 page 75 DO: I will be able to explain connections within the overall concept of Energy and then explain the relationships as well as unique characteristics the forms and types of energy. ANSWER THE EQs: How does energy connect with concepts from within our study of matter? How does energy connect with concepts in biology specifically ecological systems? How does energy connect with concepts within astronomy? Take your Homework Out to be checked. Review: Explain the PE and KE of the ball at 1,2,3, and 4. Write a sentence Summarizing the relationship of Potential and Kinetic energy. What is the relationship between Potential and Kinetic energy? 1 This point has the highest potential energy and NO kinetic energy. 2 This point has more potential energy and less kinetic energy. 3 This point has potential energy and kinetic energy. 4 This point has lowest energy and HIGHEST kinetic energy. What next… Look at the two-step transformation: 1. First identify what is happening in the current system and explain it in your journal. 2. Then create an ending to the system. 12 Dashing-KM; Snow- T: endothermic, GP; Horse- KM, KT, CP, KS,KF, GP; Sleigh-GP, CP, & KF. KINETIC POTENTIAL Mechanical = KM Gravitational = GP Radiant = KR Elastic= EP Sound= KS Nuclear = NP Friction= KF Chemical = CP Electricity = KE Thermal = T endothermic or exothermic Homework 10-16-15 Finish the transformations of energy on the second verse of Jingle Bells. Closure: •Explain how the system shown works. •What other concepts could this relate to? Lava Lamp Heat element is at the bottom of device.