Bonding: Molecular Geometry: VSEPR
advertisement

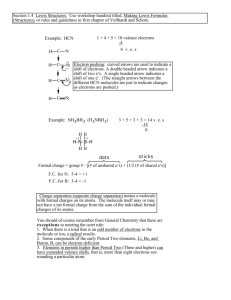
Chemistry Name: § 16.05 Bonding: Molecular Geometry: VSEPR Most Important Idea: Background The geometry of a molecule is useful in predicting their properties. For example, polar molecules dissolve polar particles (‘like dissolves like’); nonpolar molecules do not dissolve in polar substances. For example: oxygen in water; oil in water; ammonium (NH4+) in water. In a polyatomic molecule, the geometry of a molecule is determined by separating electrons as far apart as possible. (Reduces repulsion between electrons.) The method used to determine the molecular geometry is call the Valence-shell electron-pair repulsion (VSEPR) model. An atom bonded to the central atom has some attraction to nearby electrons because the atom has a positive nucleus. Nonbonding (lone pair) electrons only repel other electrons because they do not have a nucleus to attract nearby electrons. Model Water H2O AB2E2 Problems Determine the molecular geometries for each of the following molecules. Write the formula, draw the Lewis structure, determine the VSEPR model (e.g., AB2, AB4), and draw the angles between the bonded atoms. 1. H2 2. SO2 3. SO2Cl2 4. H3COH 5. CH3OCH3 7. SF4 (see below) 8. BF3 (see below) 6. HCN BF3 Document1 SF4 Chemistry 1. H2 2. SO2 3. SO2Cl2 4. H3COH 5. CH3OCH3 6. HCN 7. SF4 (see below) 8. BF3 (see below) Document1 Chemistry Document1