Separation of Pigments by Paper Chromatography
advertisement

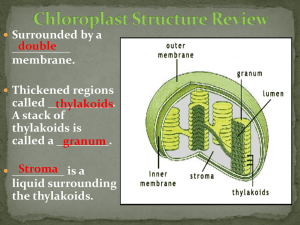
Separation of Pigments by Paper Chromatography All living organisms require energy for their metabolic (chemical) processes. The ultimate source of this energy is the sun. Photosynthetic organisms, including plants, protists (singlecelled organisms), and blue-green algae (cyanobacteria), convert light energy into the chemical energy of sugars, which can be used to power metabolism. During photosynthesis, molecules referred to as pigments (due to the wavelength, thus color, they reflect) are used to capture light energy. Four primary pigments of green plants can easily be separated and identified using a technique called paper chromatography. These pigments include two greenish pigments called chlorophylls and two yellowish pigments called carotenoids. Pigments are separated according to differences in their relative solubilities. In order to extract these pigments from the thylakoid membranes of the chloroplasts, the organelles in which photosynthesis occurs, fresh, ground or torn leaves (preferably spinach) may be soaked in acetone or concentrated alcohol. The chloroplast pigment extract pictured at left was obtained by boiling fresh leaves of spinach in 95% ethanol for several minutes and then filtering using gravity filtration. Pigments are then "painted" onto strips of chromatography paper with V-shaped tips using a small, hollow glass tube or a small paintbrush. For best results, allow the line of pigments to dry, then repeat the process until a dark green line of pigments is evident (about six times is sufficient to achieve a dark pigment line). See photo at left for technique. Next, chromatography solvent is used to separate the mixture of pigments painted on the paper. In the experiment pictured at left, the solvent used was comprised of nine parts petroleum ether and one part acetone. A small amount of this solvent is added to a large test tube and capped with a rubber stopper. Note that chromatography solvent is highly volatile and flammable. The Vshaped tip of the paper is placed in the chromatography solvent and acts as a wick to draw the solvent up the paper, separating pigments according to their relative solubility and molecular weights. The paper is allowed to remain in the solvent until the uppermost pigment band nears the top of the paper. This photograph shows the four main pigments separated from green plants using paper chromatography. The primary pigments in green plants are chlorophylls, represented by chlorophyll a and b, which appear green. Visible light, or white light, is made up of the colors of the rainbow. Some of these colors are absorbed ("used") by pigments and others are reflected. Pigments appear the color of the reflected light, so the chlorophyll pigments do not use the green portion of the spectrum. The other two pigments are types of carotenoids, which appear yellow, orange, or brown. The top band of pigments in the separation are carotenoids called carotenes, most likely beta-carotene, and appear yellowish-orange. The second type of carotenoid separated in the experiment are xanthophylls, which appear bright yellowish and are most likely lutein. The "loading line" is the location of the original pigment line painted on the paper.