BIG TEST ON THUR/FRI!
advertisement
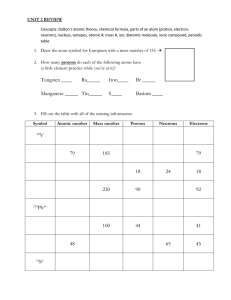
SPONCH What is SPONCH? TEXT SUPPORT 2-1 SPONCH 6 most important elements to life • • • • • • S= Sulfur P= Phosphorus O= Oxygen N= Nitrogen C= Carbon H= Hydrogen Matter • Anything that occupies space and has mass Element • Simplest form of matter, cannot be broken down chemically into a simpler kind of matter Periodic Table of Elements • Organized table of elements discovered so far • Organized according to atomic structure and chemical characteristics Atoms and Atomic Structure • Atoms are the simplest form of an element that keeps all the properties of the element Model of the Atom • Parts of the atom: – Protons (+), Neutrons and Electrons (-) – Nucleus: central core of the atom that contains • Protons • Neutrons – Electrons orbit the nucleus Determining Atomic Structure Using the Periodic Table • Atomic number = # of protons and is smaller number by the symbol • Atomic mass number = # of protons + # of neutrons • Assume for now that protons =electrons Practice Element hydrogen helium carbon oxygen sodium chlorine argon sulfur # protons 1 # neutrons 0 # electrons 1 Practice Element hydrogen helium carbon oxygen sodium chlorine argon sulfur # protons 1 2 6 8 11 17 18 16 # neutrons # electrons 0 1 2 2 6 6 8 8 12 11 18 17 22 18 16 16 Types of Bonds • IONIC- strong attraction between elements due to opposite charges • COVALENT – bond formed between elements by sharing electrons(weaker than ionic) • HYDROGEN – weakest type of bond. This is a weak force between molecules as opposed to within a molecule. Ionic • One element gains electrons becoming ____ • The other element loses electrons becoming _____________ • Opposites attract • Most Ionic compounds Dissolve easily in water – EX. NaCl Covalent • Sharing of electrons to fill the valence shell • Examples– methane (CH4) and carbon dioxide (CO2) Hydrogen bonds • Caused by partial positive and negative charges • Water is best example Hydrogen Bond ﮦ+ ﮦ- How does salt dissolve in water? • Na+ and Cl – • Ions become attracted to the partial charges on water WATER CHEMISTRY 2-2 CHEMISTRY of WATER Acids, Bases and Buffers Structure and geometry of water Four hydrogen bonds PROPERTIES OF WATER • Cohesion and adhesion • Universal solvent Adhesion and Cohesion • Adhesion – H bonds on water molecules attract to surfaces of another substance ex – causes meniscus shape in grad. cyclinders Universal Solvent Solutes that dissolve in water • Hydrophilic – water loving substances – ions, salts, polar molecules • Hydrophobic – water fearing – oils, hydrocarbon chains, – Nonpolar molecules Dissociation of Water pH Scale Chemistry Basics Quiz Element Ato Atomic # of mic Number protons Mas s Li Lithium He Helium B Boron Na Sodium # of # of neutrons electrons Draw the following and label as atom or compound • Carbon • Water Explain the type of bond(s) formed to make a water molecule CARBON – the building block of life! • DRAW Carbon Carbon is able to covalently bond with up to four other elements or form double and triple bonds with other carbon atoms. Carbon • Carbon chains make up many structures of living organisms. • Varying carbon chains’ structure and/or adding various atoms and molecules to the carbon chain will change it’s function • MAKE CH4 * MAKE C2H6 * MAKE C2H4 Group of 4 Make… • C4H10 – two different ways Ring Forms of Carbons • Make C4H8 with NO DOUBLE BONDS FUNCTIONAL GROUPS • Add a hydroxyl group (-OH) to a 2 carbon chain •You just made ethanol – an alcohol that destroys liver cells MACROMOLECULES • 4 Large Molecules Important to Life – Carbohydrates – Lipids – Proteins – Nucleic Acids Stuff to know! Chapter 2-1 •Atomic # •Atomic mass •Atomic structure •Ionic bonds •Covalent bonds •Hydrogen bonds •Water chemistry –Solutions,Solvents,pH – polarity Chapter 2-3 •Carbon chem •Carbohydrates •Lipids •Proteins •Nucleic Acids CARBOHYDRATES (CH2O)n •Functions= provides energy (glucose is energy source for cells • Monomers = monosaccharides – Examples = glucose, fructose and galactose (all 3 = C6H12O6 so they are isomer) • Two linked = disaccharides – Examples = sucrose (glucose and fructose) and lactose • Polymer = polysaccharides – Examples = glycogen (animals) starch (plants) Why “bulk-up” on carbs? Why not eat carbs? CONDENSATION REACTION HOW WOULD THIS GET BROKEN DOWN? H20 HYDROLYSIS PROTEINS • Monomers = amino acids • All amino acids have – Amine group (NH2) – Carboxyl group (COOH) • R-groups differ Dipeptide FUNCTIONS of PROTEINS • • • • • Structural Hormones Transport Histones ENZYMES!!! Lock and Key Model What symptoms would you have if you had sickle cell anemia? 1 amino acid is wrong in the hemoglobin sequence = mis-shaped RBCs LIPIDS • MONOMERS = fatty acids • Saturated • Unsaturated COMPLEX • TRIGLYCERIDES • PHOSPHOLIPIDS • WAXES FUNCTIONS • TRIGLYCERIDES – insulation and energy storage • PHOSPHOLIPDS – main component in cell membranes HARDENING OF THE ARTERIES • Fats such as cholesterol and saturated fatty acids build up in arteries • What other factors contribute to arteriosclerosis? Concept Map Section 2-3 include that consist of that consist of that consist of that consist of which contain which contain which contain which contain Concept Map Section 2-3 Carbon Compounds include Carbohydrates Lipids Nucleic acids Proteins that consist of that consist of that consist of that consist of Sugars and starches Fats and oils Nucleotides Amino Acids which contain which contain Carbon, hydrogen, oxygen Carbon, hydrogen, oxygen which contain which contain Carbon,hydrogen, oxygen, nitrogen, phosphorus Carbon, hydrogen,oxygen, nitrogen, Section Outline Section 2-2 • 2–2 Properties of Water A.The Water Molecule 1. Polarity 2. Hydrogen Bonds B.Solutions and Suspensions 1. Solutions 2. Suspensions C.Acids, Bases, and pH 1. 2. 3. 4. The pH Scale Acids Bases Buffers Hydrogen bonds • Caused by partial positive and negative charges • Water is best example ﮦ+ ﮦ- Figure 2-9 NaCI Solution Section 2-2 ClCl- Na+ Na+ Water Water Figure 2-9 NaCI Solution Section 2-2 ClCl- Na+ Na+ Water Water pH Scale Section 2-2 Increasingly Basic Oven cleaner Increasingly Acidic Neutral Bleach Ammonia solution Soap Sea water Human blood Pure water Milk Normal rainfall Acid rain Tomato juice Lemon juice Stomach acid H2O sometimes breaks down into H+ and OH- Interest Grabber continued • 1. What are the reactants when wood burns? 2-4 •Section Reactants are oxygen and cellulose. • 2. What are the products when wood burns? • Products are carbon dioxide and water • 3. What kinds of energy are given off when wood burns? • Light and heat are given off. Some students may also mention sound (the crackling of a fire). • 4. Wood doesn’t burn all by itself. What must you do to start a fire? What does this mean in terms of energy? • To start a fire, you must light it with a match and kindling. You are giving the wood some energy in the form of heat. • 5. Once the fire gets started, it keeps burning. Why don’t you need to keep restarting the fire? • Once the fire gets going, it gives off enough heat to start more of the wood burning. Section Outline Section 2-4 • 2–4 Chemical Reactions and Enzymes A.Chemical Reactions B.Energy in Reactions 1. Energy Changes 2. Activation Energy C. D. Enzymes Enzyme Action 1. The Enzyme-Substrate Complex 2. Regulation of Enzyme Activity Effect of Enzymes Section 2-4 Reaction pathway without enzyme Activation energy without enzyme Reactants Reaction pathway with enzyme Activation energy with enzyme Products Figure 2-19 Chemical Reactions Section 2-4 Energy-Absorbing Reaction Energy-Releasing Reaction Activation energy Products Activation energy Reactants Reactants Products Figure 2-19 Chemical Reactions Section 2-4 Energy-Absorbing Reaction Energy-Releasing Reaction Activation energy Products Activation energy Reactants Reactants Products Enzyme/Substrate Complex CATALASE AND H2O2 You cut yourself – mom pours hydrogen peroxide on the cut. What happens? Why? Equation: More examples of enzymes and substrated ENZYME SUBSTRATE Lactase Lactose Sucrase Amylase Bromelin Pectin ENZYMES ARE PROTEINS Explain this graph Explain this graph