346N_No03_HVAC_psychrometrics
advertisement

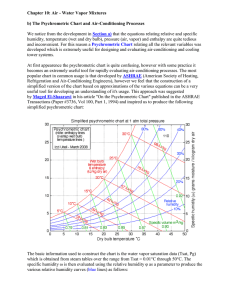
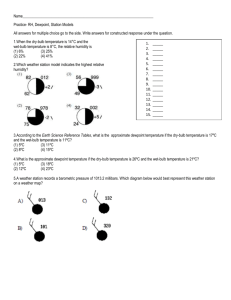
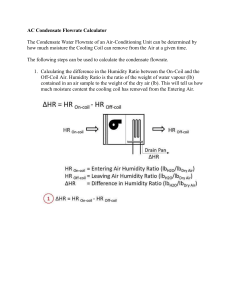
Objectives • Define psychrometric quantities • Continuation from previous class • Draw psychrometric processes • Relate psychometric variables to human comfort • Provide examples how to use psychrometric chart Psychrometric Chart • Need two quantities for a state point • Can get all other quantities from a state point • Can do all calculations without a chart • Often require iteration • Many “digital” psychrometric charts available • Can make your own • Best source is ASHRAE Fundamentals (Chapter 6) Temperature • • • • Absolute Temperature (T) (K, R) Dry-bulb temperature (t) [°F, °C] Wet-bulb temperature (t*) Dew-point temperature (td) • Mean radiant temperature (tr) • Operative temperature (to) • Effective temperature (ET*) Which temperature do you expect to be higher? A. Wet-bulb B. Dry-bulb Wet-bulb temperature (t*) • Temperature measured by a psychrometer • Lower than dry-bulb temperature • Evaporating moisture removes heat from thermometer bulb • The higher the humidity • Smaller difference between wet-bulb and dry-bulb temperature Dew-Point Temperature, td • Define temperature at which condensation happen • td is defined as temperature of that air at saturation • i.e. RH = 100% • Surfaces below the dew point temperature will have condensation • Measured with a chilled-mirror apparatus Enthalpy, h or H • Sensible – energy associate with temperature change • Latent – energy associated with moisture change • Often more important than sensible • Enthalpy (from Psychrometric chart) = total energy in air [J/kg, BTU/lb] • Sensible + latent • Very valuable for calculations Which of the following statements is not true? A) Adding steam to a room increases its relative humidity B) Placing a cup of ice water in a room decreases the humidity ratio of the room’s air C) Lowering the temperature of a room increases its humidity ratio D) Lowering the temperature while increasing the humidity ratio in a room will eventually cause the air in the room to become saturated Example 1) Condensation on windows when taking a shower • How cold does it have to be outside for condensation to form on windows? – Assumption is that windows are the same temperature as outside air – 80 °F, RH = 80% Example 2) Cooling with a oversized air conditioner • How much moisture is removed? • A central air conditioner fan blows 1500 CFM of 80 °F air @ 50 % RH past a coil. • The thermostat is satisfied when the air coming off the coil reaches 65 °F. Changing the mass of water in an air sample always A. Causes you to move vertically on the psychrometric chart B. Changes the absolute humidity of the sample C. Changes the relative humidity of the sample D. Causes you to move horizontally on the psychrometric chart E. A. and B. Other Temperatures • Not on psychrometric chart because they are environment specific • Important for comfort • Typically calculated, not measured • Often combine many factors into one parameter Comfort temperatures • Mean Radiant Temperature , tr • Temperature of an imaginary enclosure that would cause the same radiant heat loss as the present environment • Operative Temperature, to • Temperature that is weighted average of dry bulb and mean radiant temperature • Effective Temperature, ET* • Temperature at 50% RH that yields the same heat loss as for the actual environment • Environments at the same ET* should have the same comfort response 1993 ASHRAE Comfort Zone 1997/2001 ASHRAE Comfort Zone ASHRAE Comfort Zones • Still a matter of some debate • Implications of use in design: • Check to make sure that you are using the most recent version If you know the dew point temperature (td) and the dry bulb temperature (t) for a sample of air A) You can’t get the statepoint because the problem is overspecified (you know the RH = 100%, t and td). B) You get the state point by the intersection of the t and td lines. C) You get the state point by moving horizontally from td until you intersect the t line D) You get the state point by moving vertically from td until you intersect the t line Psychrometric Charts 1. Make sure chart is appropriate for your environment 2. Figure out what two quantities you know 3. Understand their slopes on the chart 4. Find the intersection • Watch for saturation