(14) SCH4C Dec 1st to Dec 5th 2014
advertisement

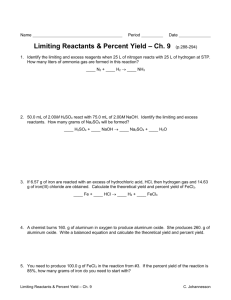
(14) SCH4C – Quantitative Chemistry Unit – Dec 1st to Dec 5th 2014 (FLIP WEEK) Date Mon Dec 1st Topic/Activities Homework Tues Dec 2nd Limiting and Excess Reactants 1. Define limiting and excess reactants 2. Thought Lab – The Limiting Item 3. Limiting Reactant (interactive) Learning Goals Success Criteria WORK PERIOD! Chemical Quantities Problem Set 2 of 3 Due Wednesday Dec 3rd p. 153 Practice UC # 1, 2, 3, 4 I will be able to explain the concept of a limiting reagent in a chemical reaction, using examples of chemical processes from everyday life (e.g. synthesis of aspirin, synthesis of ammonia) Wed Dec 3rd Percentage Yield 1. What is percentage yield? 2. Define theoretical and actual yield 3. Four factors that influence percentage yield 4. How to calculate percentage yield? pp. 158 – 159 Practice UC # 1, 2, 3, 4 I will be able to describe some possible sources of experimental error in an investigation of a chemical reaction, and explain how the errors would affect the percentage yield of products and reactants Thurs Dec 4th LAB: Percentage Yield (Day 1 of 2) Prepare and conduct “The Percentage Yield of a Chemical Reaction” experiment (p. 160 of Chemistry 12 College Preparation) Percentage Yield Lab DUE Tuesday December 9th Fri Dec 5th LAB: Percentage Yield (Day 2 of 2) Determine your mass of dry precipitate Complete analysis questions Percentage Yield Lab DUE Tuesday December 9th I will be able to conduct an inquiry to determine the actual yield, theoretical yield, and percentage yield of the products of a chemical reaction (e.g. a chemical reaction between aluminum and copper(II) chloride, assess the effectiveness of the procedure, and suggest sources of experimental error. I will be able to use qualitative observations of a chemical reaction to identify the chemical changes, presence of limiting reagents, and the products occurring in a chemical reaction. I will be able to conduct an inquiry to determine the actual yield, theoretical yield, and percentage yield of the products of a chemical reaction I will be able to use qualitative observations of a chemical reaction to identify the chemical changes, presence of limiting reagents, and the products occurring in a chemical reaction. determine the limiting reagent when given the amounts of reactants predict the amount of product formed in a reaction based on the amount of the limiting reagent determine the limiting reagent when given the masses of reactants predict the mass of product formed in a reaction given the mass of the limiting reagent identify the theoretical yield as the quantity of product as predicted by the stoichiometry of the chemical equation identify the actual yield of a reaction as the actual quantity of product collected compare the actual yield with the theoretical yield and suggest possible causes for any differences between them calculate the percentage yield of a reaction given the amounts or the masses of reactants and the actual yield of the desired product identify a limiting reagent based on observations calculate theoretical yield and percentage yield from experimental data conduct an experiment to quantitatively collect the product formed identify a limiting reagent based on observations calculate theoretical yield and percentage yield from experimental data conduct an experiment to quantitatively collect the product formed