Analysis of fruit juices_2
advertisement

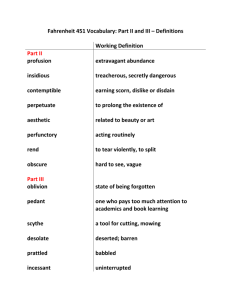
Analysis of fruit juices_2 Determination of saccharin Objective: To determine the amount of saccharine which may be added to fruit juice Introduction & principle Saccharin (o-benzoic acid sulphamide), MW=183.19, is weakly acidic & is artificial sweating agent and can be used as preserevative It has the following structure _It is used as a sucrose substitute because of its sweetness & may be present in fruit juices as an adulterant. _At neutral PH, saccharin is neutral & quite soluble in diethyl ether. The method used here for its determination involves extraction into diethyl ether, followed by titration with 0.05M NaOH, using Bromothymol blue as indicator . The latter is yellow in acid solution & blue in alkaline solution. Method 1. Extract 50g of the sample three times with 12.5 ml diethyl ether in a separatory funnel 2. Remove the lower layer & label it as (washing 1) 3. In the separatory funnel wash the diethyl ether extract with three 2.5ml portions of distilled water 4. Remove the lower layer & label it as (washing 2), & transfer the upper layer to a beaker & will be your main extract 5. Shake the combined washing with 5ml diethyl ether & the upper layer to your main extract 6. Evaporate the ether on warm water bath, dissolve the residue in 5ml acetone & evaporate to dryness, add 4ml d H2O 7. Titrate with 0.05M NaOH using Bromothymol blue indicator 8. Calculate the saccharine present : 1 ml of NaOH = 0.00916 g saccharine Wt of saccharine (g)= ml * 0.00916 % of saccharine= wt of saccharine/ wt of sample *100 Normal range 5% Determination of ethanol in soft drink Objective To determine the presence of ethanol Introduction & principle Soft drink are commonly available in markets, these drinks must be completely free from any amount of ethanol. The presence of ethanol lowers the specific gravity. If water is added to the sample, mixed & distilled, water evaporates together with the volatile materials, ethanol. So the determination of specific gravity of the distillate can detect the presence of ethanol especially if it is lower than one . Method 1. Weight 100ml of sample & add to it 50ml distilled water 2. Distil & collect 100ml of solution 3. Measure the specific gravity of the solution using S.G bottle 4. Alcohol is present if the S.G was lower than one.