Essential Cell Biology
advertisement

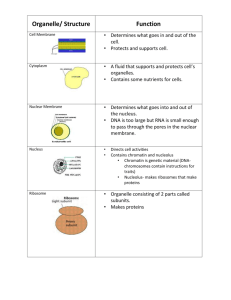
Essential Cell Biology Third Edition Chapter 15 Intracellular Compartments and Transport Hilary Truchan truchanhk@vcu.edu Copyright © Garland Science 2010 What we see in most textbooks: and what it really looks like: Figure 15-1 Essential Cell Biology (© Garland Science 2010) The real numbers Table 15-2 Essential Cell Biology (© Garland Science 2010) Overview • Briefly describe membrane-enclosed organelles of eukaryotic cells and their functions • Discuss how the protein composition of each organelle/compartment is formed and maintained • Discuss how organelles communicate with each other • Vesicles Membrane-Enclosed Organelles Internal Membranes Create Enclosed Compartments and Organelles - Segregating Metabolic Processes Examples: - Separate glycolysis from glycogenesis - Separate synthesis of protein bonds from hydrolysis of protein bonds Figure 15-1 Essential Cell Biology (© Garland Science 2010) Basic set of Organelles Found in Most Animal Cells Figure 15-2 Essential Cell Biology (© Garland Science 2010) Functions of Organelles Table 15-1 Essential Cell Biology (© Garland Science 2010) Evolutionmembranes of the ER and may Nuclear Membranes Intracellular have evolved from invagination of the plasma membrane Single compartment Plasma membrane invaginated forming a twolayered envelope of membrane surrounding the DNA Endomembrane System Figure 15-3 Essential Cell Biology (© Garland Science 2010) Protein Sorting How are proteins sorted into discrete locations? • Cell must duplicate its membrane-enclosed organelles before dividing • Most organelles formed from preexisting organelles then divide and are distributed between daughter cells • Non-dividing cells continuously generate proteins and replace proteins that have been degraded • Proteins need to be sorted correctly – organelle membrane proteins, organelle lumen proteins, secreted proteins – HOW? 3 Mechanisms How can proteins cross a phospholipid bilayer? 1. Nuclear Pores 1. Cytosol into the Nucleus – Nuclear pores - penetrate the inner and outer membranes – Selective gates – Transport specific molecules – Allow passive diffusion of smaller molecules Figure 15-5 Essential Cell Biology (© Garland Science 2010) How can proteins cross a phospholipid bilayer? 2. Protein Translocators 2. Cytosol into ER, mitochondria, chloroplast – Transported across membrane by protein translocators - located in the membrane – Protein usually has to unfold to snake through the membrane – Similar to bacteria Figure 15-5 Essential Cell Biology (© Garland Science 2010) How can proteins cross a phospholipid bilayer? 3. Transport Vesicles 3. ER onward and from one compartment of endomembrane system to another – Transport vesicles - loaded with cargo of proteins from the interior space of one compartment – Discharge cargo into second compartment by fusing with the membrane – Membrane components also delivered (lipids and proteins) Figure 15-5 Essential Cell Biology (© Garland Science 2010) Signal Sequences - direct proteins to correct organelle Localization sequences Conserved AA sequence that acts as a molecular “address” telling the cell where this protein needs to live in the cell! Table 15-3 Essential Cell Biology (© Garland Science 2010) If localization signals are removed, the protein does not arrive at the required destination Figure 15-6 Essential Cell Biology (© Garland Science 2010) How do Proteins Enter the Nucleus? Architecture of the nucleus Nuclear envelope - defines nuclear compartment - formed from two concentric membranes Inner nuclear membrane - contain proteins that act as binding sites for the chromosomes and provide anchorage for the nuclear lamina Nuclear lamina - protein filaments that provide structural support for the nuclear envelope Outer nuclear membrane - membrane similar composition as the ER membrane (continuous with) Nuclear pores - form the gates which all molecules enter or leave the nucleus Figure 15-7 Essential Cell Biology (© Garland Science 2010) Nuclear Pore Complex – A Gate Nuclear pores contain ~30 different proteins H2O filled passages - small water-soluble molecules can pass freely between nucleus and cytosol Jumble meshwork of proteins inhibit larger molecules from passing through the pore Nuclear Localization Sequence (NLS) Larger molecules need an NLS to pass through the pore - 1 or 2 short sequences of positively charged lysines or arginines Figure 15-8a Essential Cell Biology (© Garland Science 2010) EM of Nuclear Pores EM - side view of two nuclear pore complexes EM - face-on view of nuclear pore complexes Figure 15-8b Essential Cell Biology (© Garland Science 2010) Nuclear transport receptors actively transport proteins through nuclear pores NTR - grab onto sequences within the tangle of nuclear pore to carry the cargo into the nucleus Figure 15-9 Essential Cell Biology (© Garland Science 2010) Importing Proteins into the Nucleus Requires Energy - GTP hydrolysis Similar process used to carry mRNA out of the nucleus in the cytoplasm Proteins remain in fully folded conformation! Different than transport mechanisms into other parts of the cell. Figure 15-10 Essential Cell Biology (© Garland Science 2010) How do Proteins Enter the Mitochondria and Chloroplasts? © Sarah E Golding PhD. Proteins unfold in order to enter Mitochondria and Chloroplasts HELPED BY CHAPERONES TO TRANSFER AND RE-FOLD! Proteins that enter have an N-terminal signal sequence (red) Proteins translocate across both membranes simultaneously at specific sites Proteins are unfolded as they are transported Signal sequence removed after translocation completed Figure 15-11 Essential Cell Biology (© Garland Science 2010) How do Proteins Enter the Endoplasmic Reticulum? ER - most extensive membrane system in a eukaryotic cell • Entry point for proteins destined for other organelles as well as the ER itself • Proteins destined for the Golgi apparatus, lysosomes, endosomes, cell surface all first enter the ER from the cytosol • Once in ER proteins do not return to the cytosol but rather travel via vesicles Figure 15-12 Essential Cell Biology (© Garland Science 2010) © Sarah E Golding PhD. 2 kinds of proteins transferred from the cytosol to the ER 1. Water-soluble proteins - translocated across ER membrane into the ER lumen • Destined for secretion or for the lumen of an organelle 2. Prospective transmembrane proteins partially translocated across ER membrane and become embedded in it • • Destined to stay in ER membrane, membrane of another organelle, or plasma membrane Directed to ER by ER signal sequence - 8 or more hydrophobic amino acids Proteins that enter the ER begin to enter the ER membrane before the polypeptide chain has been completely synthesized Figure 15-13 Essential Cell Biology (© Garland Science 2010) ER localization signals are recognized by SRPs ER signal sequence guided to the ER membrane by: 1. A signal-recognition particle (SRP) - in the cytosol binds to the ER signal sequence when it is exposed on the ribosome 2. An SRP receptor - embedded in membrane of the ER - recognizes the SRP Figure 15-14 Essential Cell Biology (© Garland Science 2010) Soluble proteins cross ER to enter lumen • ER signal sequence - usually N-terminus - functions to open channel • Remains bound to channel as remainder of protein chain threaded through membrane as a large loop • ER signal cleaved once proteins have crossed! Figure 15-15 Essential Cell Biology (© Garland Science 2010) How are Transmembrane Proteins Transported into the Membrane? Single-pass Transmembrane Proteins ER signal cleaved once proteins have crossed! Figure 15-16 Essential Cell Biology (© Garland Science 2010) Double-pass Transmembrane Protein Start transfer-sequence - internal signal sequence used to start the protein transfer - never removed from peptide! Figure 15-17 Essential Cell Biology (© Garland Science 2010) Multi-pass Transmembrane Proteins Need additional pairs of stop and start sequences – One sequence reinitiates translocation further down peptide chain – The other stops translocation and causes polypeptide release and so on – Stitched into membrane as they are synthesized Vesicular Transport http://www.youtube.com/watch?v=rvfvRgk0MfA Vesicular Transport • Two types – secretory pathway and endocytic pathway • Secretory pathway – Entry into the ER is the first step - pathway to another destination – Initial destination is the Golgi apparatus – From Golgi to other compartments - carried out by budding and fusion of transport vesicles – Extend outward: ER plasma membrane • Endocytic pathway – Extend inward: plasma membrane lysosomes – COMMUNICATION between cells! Secretory and Endocytic Pathways Secretory pathway - RED arrows Endocytic pathway - GREEN arrows Figure 15-18 Essential Cell Biology (© Garland Science 2010) Vesicle Budding – Assembly of a Protein Coat • • • • Vesicles that bud from membranes have a distinctive protein coat on cytosolic surface coated vesicles After budding from parent organelle - sheds the coat allowing the vesicle to interact directly with the membrane it will fuse to Cells produce different types of coated vesicles – focus on Clathrin Two functions of the coat: 1. Shapes the membrane into a bud 2. Helps capture molecules for onward transport Clathrin coated vesicles http://www.youtube.com/ watch?v=eRslV6lrVxY Figure 15-19b Essential Cell Biology (© Garland Science 2010) Clathrin forms a “cage” to carry vesicles to the membrane • Clathrin - best studied vesicles have coats made largely of clathrin • Bud from the Golgi apparatus on the outward secretory pathway • Bud from the plasma membrane on the inward endocytic pathway •Electron micrograph (EM) showing a clathrin-coated vesicle forming •Assemble into a basketlike network on the cytosolic surface of the membrane •starts shaping the membrane into a vesicle Figure 15-19a Essential Cell Biology (© Garland Science 2010) Clathrin coated vesicles transport select cargo molecules Adaptins - secure clathrin coat to vesicle membrane and help select the cargo molecules Dynamin - small GTP binding protein - assembles around neck of invaginated coated pit Causes ring to contrict - pinching off vesicle from membrane Hydrolysis of GTP and help of other proteins to pinch off vesicle Figure 15-20 Essential Cell Biology (© Garland Science 2010) Adaptin proteins are specific to destination Different adaptins - adaptins that bind cargo receptors in the plasma membrane not the same as those that bind cargo receptors in the Golgi apparatus -Reflects differences in cargo molecules from each source Table 15-4 Essential Cell Biology (© Garland Science 2010) How are vesicles transported through the cytosol? • Actively transported by motor proteins that move along the cytoskeleton • Learn More about this in Chapter 17 http://www.biozentrum.unibas.c h/research/groupsplatforms/overview/unit/schoen enberger/ Vesicle Reaches Target - Recognize and Dock with the Organelle Rab proteins - surface of the vesicle are recognized by tethering proteins on the cytosolic surface of the target membrane Specific combination of Rab proteins and tethering proteins - identify membrane type Ensure vesicles fuse only with the correct membrane SNAREs - transmembrane proteins important for docking the vesicle in place SNAREs on the vesicle (v-SNAREs) interact with complementary SNAREs on the target membrane (t-SNAREs) Vesicle Fusion - deliver its cargo and adds vesicle membrane to organelle Figure 15-21 Essential Cell Biology (© Garland Science 2010) Membrane fusion sometimes needs a specific molecular signal • Fusion requires the two lipid bilayers come within 1.5nm of each other so lipid can intermix • Need to displace water from hydrophilic surface of the membrane • After pairing the v-SNAREs and t-SNAREs wrap around each other = pulls two membranes into close proximity Figure 15-22 Essential Cell Biology (© Garland Science 2010) Intracellular bacteria • Chlamydia spp. • Mycobacterium • Salmonella • WHY? Reduced genome sizes compared to extracellular bacteria • E. coli = 4.6 megabases • Chlamydia = 1.3 megabases • Do not have the genes to synthesize many essential nutrients – e.g. Amino acids • Must parasitize these from their host! • Take advantage of vesicular trafficking and hijack nutrient-rich vesicles! Many intracellular bacteria target Rab Proteins! • Mycobacterium tuberculosis acquires iron and other nutrients by targeting recycling endosome and transGolgi Rab Proteins • Chlamydia spp. acquire sphingolipids and amino acids by targeting trans-Golgi Rab Proteins http://www.rki.de/EN/Content/Institute/Departments Units/JuniorGroups/JRG5.html Uninfected human epithelium cells (left) with compact Golgi band close to the cell nucleus (blue) and cells infected with Chlamydiae trachomatis with Golgi fragments (red) which accumulate around the bacterial inclusion (green). How do proteins cross the plasma membrane? Secretory Pathways Exocytosis • Exocytosis - newly made proteins, lipids, and carbohydrates are delivered from the ER, via the Golgi apparatus, to the cell surface by transport vesicles that fuse with the plasma membrane • FIXED sequence of membrane-enclosed compartments - often chemically modified en route Chemical modifications which occur in the ER • Disulfide bonds are formed between cysteine side chains Remember? Stabilize protein structure • Glycosylation - covalent attachment of short oligosaccharide side chains Remember? Protect extracellular proteins, serve as transport signal form carbohydrate layer, help with cell to cell recognition – Carried out by glycosylating enzymes in ER but not in the cytosol Glycosylation • Glycosylation - addition of oligosaccharide side chains at asparagine residue residues added en bloc • 14-sugar oligosaccharide is originally attached to specialized lipid in ER membrane – dolichol • Not all proteins in ER are glycosylated. Requires specific tripeptide sequenc adjacent to the modified asparagine. This is the beginning of protein modification! Proteins are further modified in the Golgi. Figure 15-23 Essential Cell Biology (© Garland Science 2010) Regulated ER Exit – protein quality control! Proteins that function in ER - have ER retention signal Exit from ER highly selective Proteins that fold incorrectly or multi-meric proteins that fail to assemble properly are retained in ER and bind to chaperone proteins Chaperone proteins holds proteins in ER until proper folding occurs - if does not occur proteins are degraded Figure 15-24 Essential Cell Biology (© Garland Science 2010) Unfolded protein response (UPR) Unfolded protein response (UPR) - when cells protein production exceeds carrying and folding capacity UPR prompts the cell to make more ER - including all molecular machinery (lots of transcription!) UPR allows cell to adjust size of ER to met the cellular needs - if misfolded proteins continue to accumulate (out of control!) - UPR can direct cell to undergo apoptosis Figure 15-25 Essential Cell Biology (© Garland Science 2010) Proteins further modified and sorted in the Golgi apparatus • Oligosaccharides added in ER are further modified – removing or adding sugars Golgi apparatus • Usually located near cell nucleus • Collection of flattened membrane-enclosed sacs called cisternae with two distinct faces: – Cis face is adjacent to the ER – Trans face points toward the plasma membrane • Outermost cisterna on each face is connected to a network of tubules and transport vesicles The Golgi apparatus! Molecular post office Proteins enter at Cis-face: move through and exit Golgi or return to ER Proteins exit at trans-face: transported to Plasma membrane or lysosomes Figure 15-26 Essential Cell Biology (© Garland Science 2010) Secretory proteins are released by exocytosis Figure 15-18 Essential Cell Biology (© Garland Science 2010) Secretory cells - Regulated and Constitutive (operates continually) Pathways Constitutive Secretion vs Regulated Secretion Constitutive secretion – all Eukaryotic cells NO Signal Sequence • Proteins incorporated into plasma membrane, extracellular matrix or are signaling molecules Regulated secretion – specialized cells need signal to stimulate fusion with the plasma membrane and release cargo to cell exterior • Example: Insulin! Figure 15-27 Essential Cell Biology (© Garland Science 2010) Insulin is released by regulated secretion Example: Release of insulin from a secretory vesicle of a pancreatic cell Signaled to release by an increase in glucose levels in the blood Figure 15-28 Essential Cell Biology (© Garland Science 2010) How do proteins enter the cell? Endocytic Pathways Review video: http://www.youtube.com/watch?v=SGBiy1HlWH8 Endocytosis • Endocytosis - eukaryotic cells continuously take up fluid, as well as large and small molecules • Material to be ingested enclosed by a small portion of plasma membrane - buds inward and then pinches off to form an intracellular endocytic vesicle 2 main types of Endocytosis • Pinocytosis (“cellular drinking”) - ingestion of fluid and molecules via small vesicles (< 150nm in diameter) – Carried out predominantly by clathrin-coated vesicles • Phagocytosis (“cellular eating”) - ingestion of large particles via large vesicles called phagosomes (generally > 250nm in diameter) – Only “phagocytic” cells do this Phagocytic cells can ingest whole cells! Figure 15-32 Essential Cell Biology (© Garland Science 2010) Macrophage engulfing a pair of red blood cells Receptor-mediated Endocytosis Pinocytosis is indiscriminate Receptor-mediated Endocytosis = controlled pinocytosis – More efficient - macromolecules bind to complementary receptors on the cell surface and enter the cell as receptor-macromolecule complexes in clathrin-coated vesicles – Selective concentrating mechanism – Increases efficiency of internalization of particular macromolecules more than 1000-fold compared to pinocytosis – Example: Cholesterol LDL enters cells by receptor-mediated endocytosis Figure 15-33 Essential Cell Biology (© Garland Science 2010) Endosomes – site for sorting of imported macromolecules • Two sets of endosomes – Early endosomes: Just beneath plasma membrane mature into late endosomes as they fuse with each other – Late endosomes: Closer to the nucleus • Interior of endosome kept acidic by proton pump in the endosomal membrane – pH 5-6 – Low pH causes receptors to release their cargo • Main sorting station in the inward endocytic pathway – trans-Golgi is the sorting station for exocytic or secretory pathway! Routes taken by receptor once they enter the endosome differ according to the receptor type 1. Recycling 2. Lysosomes 3. Transcytosis Figure 15-34 Essential Cell Biology (© Garland Science 2010) Lysosomes are the Principal Sites of Intracellular Digestion Lysosome contains hydrolytic enzymes and a proton pump Digest worn out organelles and extracellular materials pH ~5 (acidic) Unique membrane contains transporters to allow products of the digestion to be transferred to the cytosol to be either excreted from cell or used by the cell Figure 15-35 Essential Cell Biology (© Garland Science 2010) Acidic pH and destructive enzymes are contained within membrane! The lysosome - The cell dumping station! Endocytosed, phagocytosed and autophagosomal material are trafficked to the lysosome for destruction! Autophagy is the destruction of an organelle – envelopes organelle and takes it to the lysosome Figure 15-36 Essential Cell Biology (© Garland Science 2010)