Presentation on Logistics Network Map of India JETRO
advertisement

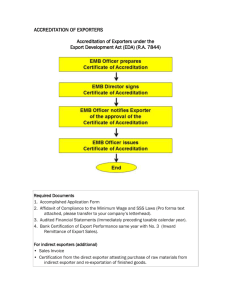
(PHARMACEUTICALS EXPORT PROMOTION COUNCIL) Set up by Ministry of Commerce and Industry GOVERNMENT OF INDIA Export Opportunities for Pharma & Herbal Industry Presentation by: G.Vijay Kumar Consultant Advisor Pharmexcil – In Service of Nation Pharmexcil is an autonomous export promotion council (EPC) set up by by Ministry of Commerce & industry, Government of India setup on 12 May, 2004 With the objective of focusing on healthcare and pharmaceutical products in the global arena 2 Pharmexcil is the designated authority for drugs & pharmaceutical sector dealing in the following products/ services from India: Drug formulations Biological products Drug intermediates Biotechnology Veterinary Drugs Herbal products Homeopathy Nutraceuticals & Phytochemicals Medicinal plants Diagnostics Surgical dressings Bulk drugs (APIs) Contract research Clinical research Contract manufacturing Collaborative research Pharma industry related services Technologies/consultancy 3 In the duty of export promotion, Pharmexcil provides the following services to Indian pharmaceutical companies: Dissemination of trade enquiries Organizing trade delegations from India to overseas markets as also reverse delegations Organizing buyers-sellers meets Organizing participation in international trade fairs Providing policy inputs to the government of India on India’s bilateral trade in pharmaceuticals 4 Dissemination of India’s pharmaceutical trade statistics Preparing technical publications, exporters’ directory, etc. Resolving grievances of pharmaceutical importers and exporters Organizing national & international seminars on areas related to pharmaceutical industry. Providing Patents related information through our Patents Facilitation Center Issuing Certificate of Origin 5 Currently Pharmexcil is among the councils with member base of 2,869 (2008-09) in the industry Large: 296 SSM: 1270 ME: 1302 Total: 2869 Which includes approx 500 herbal manufacturers from Maharashtra, Gujarat, Delhi, TN, AP 105+65+40+35+35 etc Break-up of Pharmexcil Membership Small Scale Manufactur ers, 1270, 44% Large Scale Manufactur ers, 297, 10% State-wise Composition of Pharmaceutical Members Others, 314.00, 11% Karnataka, 109.00, 4% Tamil Nadu, 164.00, 6% Merchant Exporters, 1302, 46% Maharashtra, 1,173.00, 43% Delhi, 246.00, 9% Gujarat, 352.00, 13% Andhra Pradesh, 385.00, 14% 6 The Pharma Industry of India US$ 18 billion industry Produces 70% APIs, almost all formulations within the country Very strong in Indian medicine systems of Ayurvedic, Homoepathy, Unani, Siddha and Herbals medicines Abundant scientific, technical & intellectual manpower, Excellent world-class national laboratories, specialized in development processes and cost effective technologies An efficient and cost effective source for procuring generic drugs, especially the drugs going off patent shortly. Herbal Ayurvedic Cell Pharmexcil opened Herbal Ayurvedic CELL exclusively to cater to the needs of Herbal Ayurvedics Exports – Operative from December 2006 Few salient points covered: Constituted separate Committee for Herbal/Medicinal plants involving eminent persons from the Indian industry and Academicians, which make suggestions to the Council on various issues. Efforts are made to introduce Export Certification scheme specifying the limits for heavy metals, pesticide residue, aflatoxins and bio burden. 8 Indian Pharmaceutical Industry – Key Strengths Strong manufacturing base, Strong process development skills Strong marketing and distribution network Cost competitiveness - Ranks 4th in the world, accounts 8% by vol and 2% by value. Network of laboratories and R&D infrastructure Low cost of production & Low R&D costs Highly trained pool of scientists and professionals - 15% of Indian Pharma scientists are in USA, hence good network World-class quality products Potential ground for clinical trials Fast growing health care industry Rich biodiversity Fast growing Biotech industry with US$ 2 billion market Highest Quality approvals for manufacturing facilities from USFDA, EDQM, MHRA etc. Global Pharma Markets Year 2000 2001 2002 2003 2004 2005 2006 2007 2008 Total Countries (229) Source Data : Intracen/ims health Value US $ Billions 106.51 131.75 163.80 199.39 239.27 348.62 502.65 712.00 773.00 % Growth 23.68 24.33 21.73 20.00 45.70 44.12 41.65 08.00 2008 India’s Contribution 7.3 (1% Share) Indian Pharma Export Performance Total Pharma 2003-04: 2004-05: 2005-06: 2006-07: 2007-08: 2008-09: Herbal + Medica(me)nts (ayush) Rs. 15213.24 Crs. Rs. 17857.80 Crs. Rs. 21578.96 Crs. Rs. 26895.00 Crs. Rs. 29140.00 Crs. Rs. 35402.50 Crs. 511.20 693.46 540.55 636.55 792.17 808.17 (upto Feb 2009) (upto Jan 2009) Ranks 4th in Exports “Principal Commodity” wise with 4.74% Contribution Top World Herbal Exporters 2007 Plants & pts of plants (used in pharma,perf,insect etc) S.No. COUNTRY 1. China 2. Germany 3. USA 4. Canada 5. India 6. HongKong SAR 7. Others VALUEUS $ Millions 418.24 107.00 114.00 90.51 80.00 67.22 SHARE % 23.6 7.0 6.8 6.7 5.9 4.9 35.60 Top World Herbal Importers 2007 S.No. COUNTRY 1. USA 2. China. HongKong SAR 3. Germany 4. Japan 5. France 6. Others SITC VALUEUS $ Millions 247.60 SHARE % 16.30 179.05 11.80 154.25 118.00 80.01 10.20 7.80 5.30 48.70 Indian Export of Crude Drugs Top 10 Products Rs in Crs. S. No. 1 2 3 4 5 6 7 8 9 10 COMMODITY Gaur gum CastorOil – Fractions Psyllium Husk (Isobgul) Gaurgum Refined Peppermint Oils - Mentha Hydrogenated oils Pepper Oleoresins Capsicum Oleoresins Turmeric Oleoresins Others Senna Total Exports (40 Items) 2007-2008 931.00 543.50 255.00 193.00 174.00 87.00 80.00 50.00 42.00 40.00 2882 SHARE % 35.30 20.60 10.00 7.30 6.60 3.30 3.00 1.90 1.60 1.40 100.00 Indian Export of Herbal & AYUSH Products - Country wise S.No Importing Country Value % 1 China 88.69 20.59 2 USA 87.29 20.27 3 Germany 29.16 6.77 4 Singapore 25.25 5.86 5 Russia 19.44 4.51 6 Pakistan 10.81 2.51 7 UAE 10.28 2.39 8 UK 9.89 2.3 9 Japan 9.32 2.16 9.06 2.1 430.66 100 10 Netherlands Total includes menthol exports, Value million U.S.$ Herbal Medicines & Regulatory Status – Region Wise Region American Region Eastern Mediterranean Region European Region (selected) CIS & others South East Asia WesternPacific Region African Region Regulatory 14 11 20 15 7 12 12 Non-Regulatory 4 5 1 2 3 10 24 India’s Vast Natural Resources India is one of the major raw material-producing nations of South Asia and posses about 8% of the estimated bio diversity of the world with approximately 8000 Medicinal Plants species from flowering plants available in the wild. Many species are being used in Traditional Systems of Medicine as, Ayurveda – SiddhaUnaniHomeopathyModern medicine- 7000 species 600 species 700 species 450 species 30 species Provides > 35 million workdays of employment a year. Special Export Promotion Schemes for Exporters Govt. of India extends financial assistance, through Export Promotion Councils, for the promotion of Pharma industry, under the following two schemes: Market Development Assistance (MDA) Scheme Market Access Initiative (MAI) Scheme Market Development Scheme: The Objectives of the MDA Scheme is basically to assist the SMEs to promote exports of their products and Explore new markets for their product Focus areas have been identified by the Government of India viz: Focus CIS Focus Africa Focus Asean +2 (i.e. Australia & New Zealand.) Focus LAC 17 ‘Complementary therapies’ or ‘Alternative therapies’ were not always known as complementary or alternative. In the past these were the only available therapeutic agents. Traditionally plant, sometimes animal or mineral derived products were used for therapeutic purposes. In many developing countries patient care still relies extensively on herbal or similar preparations. The World Health Organization estimates that the extent of this reliance is as high as 80%. [WHO-2004]. India’s Vast Natural Resources India is one of the major raw material-producing nations of South Asia and posses about 8% of the estimated bio diversity of the world with approximately 8000 Medicinal Plants species from flowering plants available in the wild. Many species are being used in Traditional Systems of Medicine as, Ayurveda – SiddhaUnaniHomeopathyModern medicine- 7000 species 600 species 700 species 450 species 30 species Provides > 35 million workdays of employment a year. MDA SCHEMES Objective of the MDA Scheme is basically to assist the SMEs to promote exports of their products & to Explore new markets for their product Maximum no. of permissible participations is 5(five) in a financial year i.e. one each in Focus Areas + one in General area. Company applying for MDA shall not be under investigation/charged / prosecuted / debarred / black listed under the Foreign Trade Policy of India or any other law relating to export and import business. Maximum MDA assistance shall be inclusive of MDA assistance received from all Govt. bodies / EPCs/ FIEO / ITPO etc. Member exporters of EPCs etc would also be eligible for MDA assistance for participation in events organized by ITPO abroad. Their applications / claims would be routed / reimbursed through the concerned EPC etc. A maximum of 3(three) participations in a particular trade fair / exhibition would be eligible for MDA assistance. 20 Exporting companies with an FOB value of exports of up to Rs.15.00 crores in the preceding year are eligible for MDA scheme. Assistance for Travel (economy excursion class) + built up furnished stall in Focus Areas (Assistance Amount subject to ceiling – Shown in separate slide). Assistance available for participation through Council sponsored activities. Assistance permissible to one regular employee/director/ partner/proprietor of the company. Exporter of foreign nationality or holding foreign passport will not be eligible. Intimation application must be received in any of the offices of Pharmexcil with a minimum of 14 days advance notice excluding the date of receipt of application in Pharmexcil & the date of departure from the Country. (Application available for download from our Website www.pharmexcil.com 21 Companies participating for more than 3 times including past cases for a particular fair/exhibition have to participate in that fair on self financing basis. Sl. No. Area / Sector No. of visits Maximum Financial ceiling per event Trade Fair / Exhibition Rs.in lakhs Trade delegation Rs.in lakhs 1 Focus LAC 1 1.80 1.00 2 Focus Africa (including WANA countries) 1 1.50 0.70 3 Focus CIS 1 1.50 0.70 4 Focus ASEAN + 2 1 1.50 0.70 5 General 1 0.80 Nil Total visits 5 22 After completion of the activity, the exporters have to file their claims with Pharmexcil. Claim Form and list of documents to be submitted are available in our web site www.pharmexcil.com Claims must be filed immediately upon return to India after completion of activity but positively within 45 days from their return to India. 23 MAI SCHEME Market Access Initiatives (MAI) Scheme is an Export Promotion Scheme envisaged to act as a catalyst to promote India’s export on a sustained basis. The scheme is formulated on focus product – focus country approach to evolve specific market and specific product through market studies / survey. Assistance provided to EPCs / ITPOs etc for enhancement of export through accessing new markets or through increasing the share in the existing markets. Level of assistance for each eligible activity is fixed. Reimbursement of Product Registration cost @ 50% and upto Rs 50 Lacs pa 24 Eligible activities for financial assistance under MAI are as under: 1. Marketing Projects abroad: Opening of Showrooms/Warehouses Organizing ‘Trade Festival of India Promotion of Brand India National level participation in major international Fairs Display in in International departmental stores Publication of World class Catalogues Publicity campaign and brand promotion Research and Product Development Industrial clusters for marketing abroad Reverse BSMs in India 25 2. Capacity Building Imparting training for Indian exporters Up gradation/improvements in Laboratories Quality up gradation of select products for export markets Developing Common facility centers, design centers, packing Hiring consultants in the buyer/prospective country 3. Statutory Compliances Charges for statutory compliances in the buyer country like registration charges For contesting litigation in the buyer country on anti dumping duties etc. 4. Studies Market studies/export potential studies WTO related studies Trade related studies like FDA, RDA etc Note: Assistance under MAI scheme is available to Pharmexcil for the promotion of Pharma industry as whole. Individual exporters are eligible for assistance for reimbursement of product registration charges, R&D projects for select export markets, hiring of consultants in the buyer country only 26 Market Access Initiative Scheme (contd) Statutory Compliances Charges for statutory compliances in the buyer country like registration charges For contesting litigation in the buyer country on anti dumping duties etc. Studies Market studies/export potential studies WTO related studies Trade related studies like FDA, RDA etc. For other schemes visit the sites of AYUSH NMPB APEDA Vishesh Krishi Upaj Yojana Backward Regions Grant Fund Deptt of Ayush Centrally Sponsored Scheme of QC of Ayurveda, Siddha, Unani and Homeopathy Scheme No: 4 Rs 30 lakhs or 30% of expenditure for in house QC Lab or Scheme No: 5 Assistance of Rs 30 lakhs or 30% of expenditure for up gradation of facilities to US FDA / EU Good Mfg Practices certification standards NMPB Various schemes for Cultivation of MAPs, preparation of Project Reports, Documentary Film preparation, Establishment & maintenance of Herbal Gardens & Nursery etc. (evaluated cost of about Rs. 630 lacs for 11th 5 Yr plan. Nurseries 4 – 20 Lacs Cultivation 20% - 75% (depending on the species of cultivation selected) Post Harvestment Management 5 Lacs (50%-100%) Processing & Value addition 10- 50 lacs @ 25- 50% Further details available on the NMPB web site APEDA : 50% of analytical charges are reimbursed for testing Egg powder, Honey & Grapes. Vishesh Krishi Upaj Yojana: Duty Credit at 5% of the FOB Value Backward Regions Grant Fund: Grant of Rs 10 crore per District (total 250 Districts) for providing technical assistance, office infra structure, training, conduct of surveys, etc at Panchayat level. Kerala has 2 districts which include Palakkad and Wynad. Cluster development from DST for new ideas:www.tdb.gov.in – Provides for Reimbursement of R&D Innovative activities. Activities for 2009-10 Delegations/BSM to Abroad : South Africa, Nigeria, Kenya, Libya - July 09 Vietnam, Cambodia, Philippines and Taiwan - Sep 09 Russia, Ukraine, Armenia, Belarus, Moldova - October 09/February 10 Brazil, Bolivia, Paraguay, Uruguay, Venezuela - August 09 Kuwait, Syria, Jordan, Egypt, Turkey - November 09 Hungary, Slovenia, Poland, Slovakia - January 10 Exhibitions Abroad: 1. BIO 2009, Atlanta, USA UAE May 18-21 2. Vitafood, Geneva, Switzerland 5-7 May 2009 3. CPhI China June 09 4. Pharmed & Healthcare 09, Vietnam 16-19 Sep 09 5. Apteka, Moscow or Apteka, Ukraine Oct 09 or Feb10 6. CPhI, South America, Brazil 26-28 Aug 09 7. CPhI Worldwide 09 13-15 Oct 09 8. CPhI, India, 1-3 Dec 09 9. Arab Health, 26-29 Jan 10 International Conferences India – CIS Countries – January / February 2010 30 Pharmexcil’s other activities / services Patents Facilitation Center services to the members: at Hyderabad offers following General Information on Patents, Patent status of pharmaceutical products in India and other countries, Interpretation of search information Search based intellectual property related technology and prior art search Invention mining, Patentability opinion, Guidance and opinion of IM launch Infringement analysis and opinion and Freedom to operate opinions Pharmacopoeia & Other publications available with Pharmexcil: IP 2007, Ayurvedic Pharmacopoeia of India, AFI BP 2009, BP Vet 2009, USP NF 2009 EP 2008 (EP6.0 – 6.7, Extra Pharmacopoeia 2007 Other Reference Books: Merck Index– 14th Edition, PDR-63rd Edition CCRAS Publications , 31 country reports Monographs provided at a nominal service charge 31 Offices of Pharmexcil Head Office: Pharmexcil 101, Aditya Trade Centre Ameerpet Hyderabad – 500 034. Phone: 91 – 40 – 23735462/66 Fax: 91 – 40 – 23735464 Email: info@pharmexcil.com Regional Office, Mumbai Pharmexcil T.V. Industrial Estate, Unit No. 211 2nd Floor, 248-A, S.K. Ahire Marg Worli, Mumbai - 400 030. Phone: 91 - 22 - 24938750 / 51 Fax: 91 - 22 - 24938822 Email: romumbai@pharmexcil.com Regional Office, Delhi Pharmexcil 305, Padma Tower II 22, Rajendra Place New Delhi - 110008 Phone: 91 - 11 - 41536654 / 45062550 Fax: 91 - 11 - 41536658 Email: rodelhi@pharmexcil.com 32 Requirements for Membership: 1. Import Export Code Number 2. SSI Certificate 3. Bankers Certificate 4. Drug License, 5. PAN Fees : New Renewals For details visit our Website www.pharmexcil.com Rs SSM 6000 ME 9000 LSM 28500 Rs 4500 6500 21000 Our Website - www.pharmexcil.com 34 THANK YOU 35