Oct. 19th powerpoint - The University of Texas at Dallas
advertisement

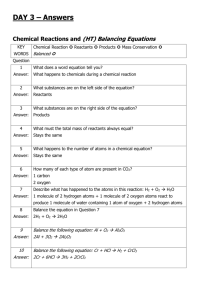
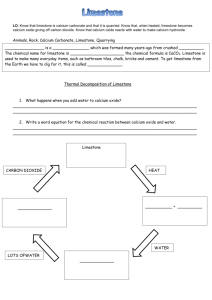
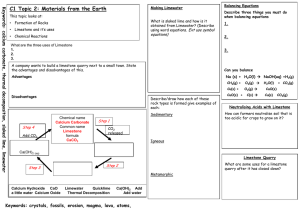
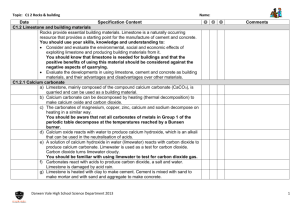
Laboratory 07 GRAVIMETRIC ANALYSIS : CALCIUM CARBONATE IN TEXAS LIMESTONE Objectives - Use an understanding of solubility rules and precipitation reactions to isolate calcium from a real world sample - Use mass relationships to determine the percent composition of calcium carbonate in limestone Background An understanding of the properties of a substance begins with the determination of its elemental composition Percent composition of a pure substance can be determined from the formula : % Composition = (mass of element) X 100 (molar mass of compound) Theoretical percent composition The percentage composition of an impure substance can be expressed as % Composition = (mass of component of interest) X 100 (total mass of sample) Gravimetric Analysis - Add a compound ( precipitating agent ) to a dissolved sample that combines selectively with the element of interest ( the analyte), causing it to form a solid precipitate of known identity and composition in order to identify what elements are present, and in what quantities. - Isolate and weigh the precipitate to quantify the analyte Gravimetric analysis is used to determine the amount of calcium carbonate ( CaCO ) in a sample of limestone 3 IMPORTANT: Do TWO trials in PARALLEL for the whole experiment Changes to procedure: Determination of carbonate in the Limestone 1.The limestone has already been broken into smaller pieces (Use about 1.5 g) 2. Dissolve the limestone in HCl. (10 – 15 minutes ) CaCO3 (s) + 2 HCl (aq) CaCl2 (aq) + H2CO3 (aq) H2CO3 (aq) CO2 (g) + H2O (l) CaCO3 (s) + 2 HCl (aq) CaCl2 (aq) + CO2 (g) + H2O (l) 3. Do gravity filtration using Whatman Filter paper 1. (15-20 minutes) - No gooch crucible Determination of calcium in the Limestone 1. About 5-6 drops of indicator (kept in stopper bottles) are enough to get the pink color. 2. After adding 3 grams of (NH4)2SO4 it is hard to see it dissolving as very quickly the precipitate appears and the solution now looks baby pink in color. (Ensure that (NH4)2SO4 is completely dissolved and no lumps are left behind) CaCl2 (aq) + (NH4)2SO4 (aq) CaSO4 (s) + 2 NH4Cl (aq) 3. Add about 20-24 ml NH3 solution. The color changes to a very very pale yellow. (Do this step in a hood) 4. Use vacuum filtration and Whatman Filter paper 2. (No gooch crucible) Weigh the empty filter paper + watch glass. Use the same filter paper for vacuum filtration. Wash the solid using water. Transfer the filter paper along with the precipitate to the watch glass and keep it for drying until the next lab in the drawer. Finish the calculation and turn in the lab report on next lab. Reflection Questions 1, 2 , 3 and 4 (should be answered at home) 5 (should be answered in the next lab) Grading Deviation ± 10% ± 12.5% ± 15% ± 20% ± 25% Points deducted None 2 points 5 points 10 points 15 points