Final Presentation (Apr. 13, 2004)
University of Pittsburgh
Senior Design - BIOE 1161
April 13, 2004
Design of a More Efficient Heat
Exchanger for Inducing Hypothermia in Neonatal ECMO Cases
Adam Abdulally
Erin Aghamehdi
Kim Albrecht
Rebecca Hrutkay
Outline
• Background
• ECMO & Hypothermia
• Overview
• Re-design
• Audience
• Device Description
• Features & Benefits
• Project Objectives
• Spring 04
• Future
• Project Management
• Design Alternatives
• Quality Systems
• Experimental Design
• SA calculations
• Technologies
• Competitive Analysis
• Testing Requirements
• Projected Methods
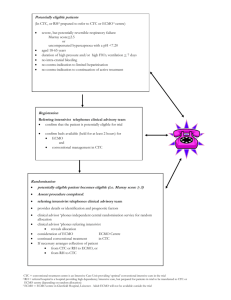
ECMO Background
• Provides temporary cardiac and/or pulmonary support for patients with potentially reversible cardiac and/or respiratory failure
• ECMO Stats:
• 8000 neonatal patients over the last
8 years
Hypothermia Background
• Patient core body temperature : 32 - 34°C
• Helps to counteract a decrease in oxygenation by reducing the brain’s oxygen requirements.
• Neurological protection for patients with:
• Sever head trauma
• Cardiac/pulmonary distress
• Other pathologies
• Current methods:
• Medications
• Ice water baths
• Cooling blankets
• IV of 4°C crystalloid
Overview
• Cooling offers a way to provide neurological protection for a patient on ECMO.
• Current heat exchangers used on ECMO are not capable of rapidly inducing patient hypothermic conditions.
• Solution:
• Design of a more efficient heat exchanger that will work in conjunction with an ECMO circuit.
Overview
• Users:
• Neonatal ECMO patients
• Customers:
• Hospitals
• Perfusionists
• Customer Requirements:
• More efficient means of inducing
Hypothermia
Overview
• Current Re-design:
• Heat exchanger is compatible with existing water bath in circuit
• Modeled similar to an oxygenator
• Increased surface area for exchange – nonporous hollow polypropylene fibers
• Priming volume maintained (60mL vs. ~65-70mL)
Features & Benefits
• Needs/requirements of end-user:
• Rapidly and accurately cools patient to desired hypothermic temperature (32-34 °C)
• Gradual rewarming of the patient’s blood <1°C/
15mins
• No clot or thromboemboli formation
• Pressure sensor on outlet of heat exchanger
• Safety of end-user:
• No significant increase in required patient priming volume
• Biocompatibility
Project Objectives – Spring 04
• Establish project objective:
• Research efficient means of cooling
• Project documents:
• DHF, scientific paper, presentation
• Heat transfer calculations:
• Energy balance equations
• Calculation of overall heat transfer coefficient (U)
• Calculation of surface area (SA)
• Calculate the number of fibers and priming volume
• Development of fiber bundles in heat exchanger
• Design of outer shell
Project Objectives –
Future Direction
• Fabrication and assembly of device
• Wet-lab testing
• Analysis
• Possible redesign of device to fulfill any unmet requirements from previous device
Project Management
•
Individual Responsibilities:
K
E
B
A
Heat Transfer
SA
Priming
Volume
Heat
Exchanger
Re-Design
Testing Analysis Paper Docs.
Accomplishments
Remaining Tasks
Device Description
• Non-porous polypropylene microfibers encased in a clear PVC housing
• Efficient heat transfer (Thermal conductivity estimated at 11.7 W/mK from the Polymer
Handbook)
• Increased SA for exchange
• Counter-current flow maximizes heat transfer
• Blood flows within the fibers
• Decrease priming volume requirement at the expense of heat transfer
Design Alternatives
• Fall 2003:
• Cooling Block
• Reasons for Rejection:
• Surface area requirement
• Spring 2004:
• Hollow 3003 anodized aluminum tubing
• Reasons for Rejection:
• Could not achieve required surface area with a reasonable amount of tubes
• Large priming volume
• Manufacturing restraints
Quality System Considerations
• Manufacturability:
• Materials:
• Polypropylene microfibers
• PVC shell
• Polyurethane epoxy
• Methods:
• Ension manufacture of fibers
• Rapid prototyping or hand machining of PVC shell
• Human Factors:
• Ensures the safety and needs/requirements of the end user are met
• Regulatory:
• Class II
• Predicate devices: All companies producing blood heat exchangers
Experimental Design
• Heat transfer within patient can be correlated by the Pennes equation:
• Mathematical modeling was used to find the desired surface area (SA)
• Incorporation of SA into heat exchanger design
• Subsequent fiber and priming volume calculations
Engineering
Technologies/Methodologies
• Solidworks
7.5 in.
Competitive Analysis
• Competitors:
• Non-invasive surface cooling
• Other heat exchanger manufacturers (Medtronic,
Gish Biomedical, etc)
• Strengths:
• More efficient heat exchange
• No additional infused liquids
• Weaknesses:
• Requires constant monitoring of patient temperature
• Requires extra pressure sensor
Constraints
• Economic:
• Costly manufacture
• Small market (~1000/year)
• Regulatory:
• Priming volume
• Biocompatible materials
Testing Requirements
• FDA Requirements:
• Preparation for Testing
• Biological Compatibility
Tests
• Physical Integrity Tests
• Performance
Characterization
Projected Methods
• Simulate a patient using a 2.5 - 5.0 L carboy
• Utilizing a full ECMO circuit
• Additional heat exchanger used for metabolic effects
• Temperature set at 37 °C
• Record heat exchange, water and blood-side pressure drops
• Perform several 6 hour trials at various water and blood flow rates
• Also test for blood damage
Acknowledgements
• Dr. Carcillo
• Mark
• Children’s perfusionists