lesson 10
advertisement

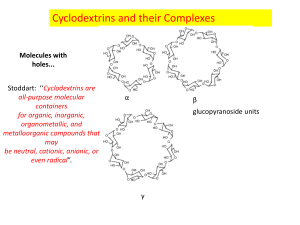
Cyclodextrin • Cyclodextrins (sometimes called cycloamyloses) make up a family of cyclic oligosaccharides, composed of 5 or more α-D-glucopyranoside units linked 1->4, as in amylose (a fragment of starch). The 5-membered macrocycle is not natural. Recently, the largest well-characterized cyclodextrin contains 32 1,4-anhydroglucopyranoside units, while as a poorly characterized mixture, even at least 150-membered cyclic oligosaccharides are also known. Typical cyclodextrins contain a number of glucose monomers ranging from six to eight units in a ring, thus denoting: • • • • • α-cyclodextrin: six sugar ring molecule • β-cyclodextrin: seven sugar ring molecule • γ-cyclodextrin: eight sugar ring molecule Cyclodextrins are produced from starch by means of enzymatic conversion. Over the last few years they have found a wide range of applications in food, pharmaceutical and chemical industries as well as agriculture and environmental engineering. It is also the chief active compound found in Procter and Gamble's deodourizing product "Febreze". History of cyclodextrins • Cyclodextrins, as they are known today, were called "cellulosine" when first described by Villiers in 1891. Soon after, Schardinger identified the three naturally occurring cyclodextrins -α, -β, and -γ. These compounds were therefore referred to as "Schardinger sugars". For 25 years, between 1911 and 1935, Pringsheim in Germany was the leading researcher in this area, demonstrating that cyclodextrins formed stable aqueous complexes with many other chemicals. By the mid 1970's, each of the natural cyclodextrins had been structurally and chemically characterized and many more complexes had been studied. Synthesis • The production of cyclodextrins is relatively simple and involves treatment of ordinary starch with a set of easily available enzymes. Commonly cyclodextrin glycosyltransferase (CGTase) is employed along with αamylase. First starch is liquified either by heat treatment or using α-amylase, then CGTase is added for the enzymatic conversion. CGTases can synthesize all forms of cyclodextrins, thus the product of the conversion results in a mixture of the three main types of cyclic molecules, in ratios that are strictly dependent on the enzyme used: each CGTase has its own characteristic α:β:γ synthesis ratio. • Purification of the three types of cyclodextrins takes advantage of the different water solubility of the molecules: β-CD which is very poorly water soluble (18.5 g/l) can be easily retrieved through crystallization while the more soluble α- and γ-CDs (145 and 232 g/l respectively) are usually purified by means of expensive and time consuming chromatography techniques. As an alternative a "complexing agent" can be added during the enzymatic conversion step: such agents (usually organic solvents like toluene, acetone or ethanol) form a complex with the desired cyclodextrin which subsequently precipitates. The complex formation drives the conversion of starch towards the synthesis of the precipitated cyclodextrin, thus enriching its content in the final mixture of products. The precipitated cyclodextrin is easily retrieved by centrifugation and is later separated from the complexing agent. Structure • Cyclodextrins, constituted only by 6-8 glucopyranoside units, can be topologically represented as toroids with the larger and the smaller openings of the toroid exposing to the solvent secondary and primary hydroxyl groups respectively. Because of this arrangement, the interior of the toroids is not hydrophobic, but considerably less hydrophilic than the aqueous environment and thus able to host other hydrophobic molecules. On the contrary the exterior is sufficiently hydrophilic to impart cyclodextrins (or their complexes) water solubility. Structure • The formation of the inclusion compounds greatly modifies the physical and chemical properties of the host molecule, mostly in terms of water solubility. This is the reason why cyclodextrins have attracted much interest in many fields, especially pharmaceutical applications: because inclusion compounds of cyclodextrins with hydrophobic molecules are able to penetrate body tissues, these can be used to release biologically active compounds under specific conditions. In most cases the mechanism of controlled degradation of such complexes is based on pH change of water solutions, leading to the cleavage of hydrogen or ionic bonds between the host and the guest molecules. Alternative means for the disruption of the complexes take advantage of heating or action of enzymes able to cleave α-1,4 linkages between glucose monomers. A unique tetramer of 4 5 -cyclodextrin–ferrocene in the solid state Fragrance-release Property of ß-Cyclodextrin Inclusion Compounds and their Application in Aromatherapy Uses • Cyclodextrins are able to form host-guest complexes with hydrophobic molecules given the unique nature imparted by their structure. As a result these molecules have found a number of applications in a wide range of fields. • Other than the above mentioned pharmaceutical applications for drug release, cyclodextrins can be employed in environmental protection: these molecules can effectively immobilise inside their rings toxic compounds, like trichloroethane or heavy metals, or can form complexes with stable substances, like trichlorfon (an organophosphorus insecticide) or sewage sludge, enhacing their decomposition. In the food industry cyclodextrins are employed for the preparation of cholesterol free products: the bulky and hydrophobic cholesterol molecule is easily lodged inside cyclodextrin rings that are then removed, leaving behind a "low fat" food. • Other food applications further include the ability to stabilize volatile or unstable compounds and the reduction of unwanted tastes and odour. The strong ability of complexing fragrances can also be used for another purpose: first dry, solid cyclodextrin microparticles are exposed to a controlled contact with fumes of active compounds, then they are added to fabric or paper products. • Such devices are capable of releasing fragrances during ironing or when heated by human body. Such a device commonly used is a typical 'dryer sheet'. The heat from a clothes dryer releases the fragrance into the clothing. References • • Villiers A., Sur la transformation de la fécule en dextrine par le ferment butyrique, Compt. Rend. Fr. Acad. Sci. 1891:435-8 Biwer A, Antranikian G, Heinzle E. Enzymatic production of cyclodextrins. Appl Microbiol Biotechnol 2002;59:609-17.