Kinetics
advertisement

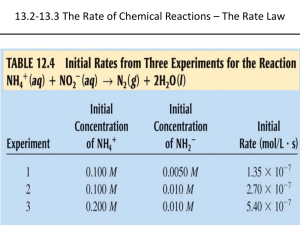
Kinetics Chapter 12 3 OUT OF 75 M/C QUESTIONS FREE RESPONSE—ALMOST EVERY YEAR Chemical Kinetics Study of Reaction rates Reaction mechanism—series of steps by which a reaction takes place Rate of Reaction the change in concentration of a reactant or product per unit time The square brackets indicate concentration of a substance. Rate = [A]t2 – [A]t1 / (t2 – t1) OR Rate = D[A] / Dt Usually considered positive even if concentration is decreasing. Example Problem Nitrogen dioxide decomposes to nitrogen monoxide and oxygen gas. At the beginning of the reaction, a pressurized 1-L flask contains 2.0 mol of nitrogen dioxide. At the end of 20 minutes, the flask contains 1.5 mol of nitrogen dioxide and 0.5 mol of nitrogen monoxide. What is the rate of the reaction in terms of M/s of nitrogen dioxide? Example Problem Solution • Important information: 2.0 mol/ L nitrogen dioxide initially After 20 minutes, 1.5 mol/L of nitrogen dioxide • Change in concentration: 2.0 mol – 1.5 mol = 0.5 mol • Change in time: 20 min • Rate: 0.5 mol / 20 min = 0.025 mol/min Average vs. Instantaneous Rate Rate is usually not constant, but decreases over time Average rate—total change over total time (like the previous example) Instantaneous rate—rate at a particular instant in time Instantaneous Rate Calculated by line’s slope tangent to the point in time on the rate curve Think about a slope: change in "y” over change in “x”—It’s the same thing as the average rate if y is the concentration and x is the time. Page 558 Rate Law A mathematical expression which This is a very important describes the dependence of concept! reaction rate on concentration of The [A] means concentration of reactants substance A. The exponent “n” is known as the order of the reaction. The n must be determined The lower case k is the Rate = k[A] order experimentally from data. rate constant. It must be lower case because k = proportionality constant upper case K is an equilibrium constant. Rate Law [A] = concentration of reactant(s) n = order of reactant Both k and n must be experimentally determined Cannot determine from balanced equation!!! Write the rate law 2NO2 Rate law always begins with “rate = k….” 2NO + O2 if NO2 is Next, put the formula for each reactant in brackets and raise it to second order the power of the order. Rate = k [NO2]2 You don’t need to include zero order reactants 2H2 + 2NO N2 + 2H2O if NO is since anything to the zero second order and H2 is first order raised power is equal to one. Combining Rate Laws Rate = D[A] / Dt n Rate = k[A] D[A] / Dt = n k[A] Since both of these are equal to the rate, they are equal to each other. More product terms can be added Use this equation to solve for either rate, the rate constant or a concentration. Example Rate = n m p k[A] [B] [C] (Greater value of exponent means greater effect on rate.) To find exponents, determine what happens if concentration of a reactant doubles. Determining orders of reactants Find two experiments in which one concentration remains the same while the other changes. Rate 2 = (D concentration)n Rate 1 Example Calculation Trial Initial Concentration of Reactants (M) [A] [B] [C] Initial Rate (M/min) 1 0.10 0.10 0.10 0.01 2 0.10 0.10 0.20 0.01 3 0.10 0.20 0.10 0.02 4 0.20 0.20 0.10 0.08 To determine orders of reactions, by method of initial rates: 1. Look at the data and find two trials that start with the same concentrations in all but one reactant. 2. See trials 1 and 2. The concentration of reactant C was doubled while A and B stayed the same. There was no effect on the rate, so reactant C is zero order. Leave it out of the rate law. Continued on next slide Example Calculation Trial Initial Concentration of Reactants (M) [A] [B] [C] Initial Rate (M/min) 1 0.10 0.10 0.10 0.01 2 0.10 0.10 0.20 0.01 3 0.10 0.20 0.10 0.02 4 0.20 0.20 0.10 0.08 3. Compare trials 1 and 3. While the concentrations of A and C stayed the same, the concentration of B was doubled and the rate doubled. Since the effect was the same, B is a first order reactant. (2 x B = 2 x rate; 21 = 2)—The exponent is the order. 4. Compare trials 3 and 4. The concentrations of B and C are the same while the concentration of A was doubled. The rate increased by a factor of 4 so A is a second order reactant. (2 x A = 4 x rate; 22 = 4)— The exponent is the order. 5.Rate = k [A]2 [B]1 Example Overall Rate: Rate = Overall 2 1 0 k[A] [B] [C] order of reaction: 2+1+0=3 Applying Rate Laws Once the rate law is established, use data to solve for k and to find rates at different conditions. Example Calculate the rate constant k for the previous reaction. Rate = k[A]2[B] Trial 1 Initial Concentration of Reactants (M) [A] [B] [C] 0.10 0.10 Initial Rate (M/min) 0.10 0.01 Example Since [C] has no effect on rate, we can leave it out when solving for k. To find the rate constant, k, choose any of the trials and substitute the concentrations and initial rate into the equation. Solve for k. Pay attention to units and include them for k. k Rate = k[A]2[B] = Rate / [A]2[B] k = .01M/sec / (.10M)2 (.10M) k = 10 M-2sec-1 Write the Rate Law Equation Look at data sets from different trials: + NH4 + NO2 N2 + 2 H2O Experiment # Initial Conc. Of NH4+ Initial Conc. Of NO2- Initial Rate (mol/L *s) 1 0.100 M 0.0050 M 1.35 x 10-7 2 0.100 M 0.010 M 2.70 x 10-7 3 0.200 M 0.010 M 5.40 x 10-7 Answer Both reactants are first order, so Rate = k [NH4+]1[NO2-]1 Solve for k. Answer Rate = k [NH4+]1[NO2-]1 Using information from trial #1: 1.35 x 10-7 M/s = k (0.100 M)(0.0050 M) k = 2.7 x 10-4 M-1s-1 2 ClO2 + 2 OH- ClO3- + H2O [ClO2] in mol/L [OH-] in mol/L 0.0500 0.100 0.100 0.100 0.100 0.0500 Initial Rate in M/s 5.75 x 10-2 2.30 x 10-1 1.15 x 10-1 Write the rate law equation and find the rate constant. Answer Rate = k [ClO2]2 [OH-] k = 230 M-2 s-1 Types of Rate Laws Differential Rate Law—expression of how rate depends on concentration (previous problems) Integrated Rate Law—expression of how concentration depends on time When one is determined, the other can be calculated. Integrated Rate Law Shows how concentration of A depends on time Usually given [A]0 and k Equation (whichever form) is in the form y = mx + b so it will be a straight line when graphed. Calculating Integrated Rate Law—First Order ln[A] = -kt + ln[A]0 ln = natural logarithm t = time [A] = conc. at time t [A]0 = conc. at time 0 Integrated Rate Law Equation Equation can also be expressed as a ratio: ln ([A]0 / [A]) = kt Half Life The time required for a reactant to reach half its original concentration t1/2 Calculating Half Life When t = t1/2, then [A] = [A]0/2 Use equation ln ([A]0 / [A]) = kt ln / ([A]0 [A]0/2) = kt1/2 So…ln(2) = kt1/2 Notice, half life does not depend on concentration. Calculating Integrated Rate Law—Second Order Rate = k[A]2 Integrated Rate Law 1/[A] = kt + 1/[A]0 Integrated Second Order Plot of 1/[A] versus time is a straight line with slope = k Half life for second order: t1/2 = 1/k[A]0 For 2nd order, half life does depend on initial concentration. Zero Order Rate Laws Often encountered when a reaction rate is limited because a surface (such as a platinum catalytic converter) is required. When surface is covered, increasing concentration of a reactant has no effect. If the reaction takes place on a surface, increasing concentration will not Zero Order Rate Laws 0 Rate = k[A] = k(2) = k Integrated Rate Law: [A] = -kt + [A]0 Half Life: t1/2 = [A]0 / 2k More Complex Rate Laws Many reactions have several reactants—each affects rate To study them, high concentrations of all but one reactant are used. Highly concentrated reactants stay practically constant, so the remaining reactant can be studied. Pseudo-First-Order If conc. of B & C are large, n m p Rate = k[A] [B] [C] = k’[A] Calculate k’ and solve for k Good Summary—Table 12.6 Importance Helps determine reaction mechanism Find rate determining step in a series of reactions so total process can be sped up Reaction Mechanisms Sum of elementary steps must give overall balanced equation Must agree with experimentally determined rate law Reaction Mechanisms Most reactions are much more complicated that they appear from their equations. Intermediate—a species that is neither a reactant nor a product but that appears and then is consumed in the course of a reaction Reaction Mechanisms Each reaction is called an elementary step. Rate of each elementary step can be written from its molecularity. Molecularity = the number of species that must collide to cause the reaction Molecularity Unimolecular—depends on one molecule; Rate = k[A] Bimolecular—depends on two molecules; Rate = k[A]2 or Rate = k[A][B] Termolecular—depends on 3 molecules; Rate = k[A]2[B] (etc) Rate Determining Step The slowest step in a reaction mechanism Determines overall rate much like pouring water through a funnel— limiting factor Rate Law Comes from the slow step and every step prior to it. Only consider species that are in the overall reaction. Only consider reactants. Species Not in Overall Reaction Intermediate—a species that is produced and then consumed in a reaction—appears in mechanism but not in overall reaction—appears as a product and then a reactant Catalyst—a species that is used during one step of a mechanism but is reproduced later—appears as a reactant and then a product Determining Mechanism See if rates of elementary steps agree with observed rate law If yes, it is an acceptable mechanism Never proven—only possibly correct Example Problem 2NO2 + F2 2NO2F Rate = k[NO2][F2] Possible Mechanism?? NO2 + F2 NO2F + F (slow) F + NO2 NO2F (fast) Example Overall Reaction: I2 + H2 2HI Step 1: I2 2I Step 2: I + H2 H2I Step 3: H2I + I 2HI Does this give the overall equation? If step one is rate determining, what is the rate law? Step 2? Identify any catalysts or intermediates. Example 2 NO2 (g) + F2 (g) 2NO2F (g) Rate Law: Rate = k [NO2] [F2] Possible Mechanism: NO2 + F2 NO2F + F (slow) F + NO2 NO2F (fast) Is this a possible mechanism? Identify any catalysts or intermediates. Example NO2(g) + CO(g) NO (g) + CO2(g) Step 1: NO2 + NO2 NO3 + NO (slow) Step 2: CO + NO3 NO2 + CO2 (fast) If the mechanism is correct, what is the rate law? Identify any catalysts or intermediates. Example Write the rate law and overall reaction: A + A + B C + D (fast) C + E D + B (slow) Identify any catalysts or intermediates. Collision Model Molecules must collide in order to react Anything that increases frequency or energy or effectiveness of collisions increases reaction rate. Effective Collisions Only a small fraction of collisions produce reactions. Many ineffective collisions occur because energy is too low or orientation is wrong. Some orientations for a collision between BrNO molecules. Orientations (a) and (b) can lead to Activation Energy The minimum amount (threshold) of energy that a system must have to produce a reaction Using k to Calculate Activation Energy k= -E /RT a zpe k = rate constant z = collision frequency p = steric factor (fraction of collisions with proper orientation) e-E /RT = fraction of collisions with enough energy to produce reaction a Arrhenius Equation -E /RT k = Ae a zp replaced by A A is the frequency factor for the reaction Ea is activation energy R is universal gas constant (8.3145) Taking ln of both sides gives a y = mx +b form line equation Arrhenius Equation ln(k) = -Ea/RT+ lnA In slope intercept form: Slope = Ea Intercept = A To Find Activation Energy Measure rate constant (k) at several temps Plot ln(k) versus 1/T To Find Activation Energy Use equation: ln(k2/k1) = Ea/R (1/T1 – 1/T2) Example Problem—p. 588 To Speed up a Reaction: Increase temperature 2. Increase pressure 3. Increase concentration 4. Increase surface area 5. Add a catalyst 1. Catalyst A substance that speeds up a reaction without being consumed in the reaction Enzymes—biological catalysts Works by providing the reaction with a new pathway with lower activation energy Effect of a Catalyst Notice that energy difference between products and reactants is unchanged. Effect of a Catalyst- Heterogeneous Catalysis Usually gaseous reactants adsorbed on a metal surface Example—hydrogenation of ethylene Breaking H-H bond requires lots of energy, but metal-H interactions weaken bond and allow bonds to break at lower energy Homogeneous Catalysis Catalyst and reactants exist in the same phases Example: Catalysis of ozone by nitric oxide and freons (CCl2F2)